The departure of the director of the Tobacco Science Office at the US Food and Drug Administration from the tobacco industry highlights a long-standing issue of the "revolving door" between government regulatory agencies and the industries they oversee.
On Tuesday, Matt Holman left the FDA to work at Philip Morris International, a company that sells products such as Marlboro cigarettes and the IQOS electronic smoking device overseas. Holman's work at the FDA had an impact on decisions related to the safety of products such as electronic cigarettes.
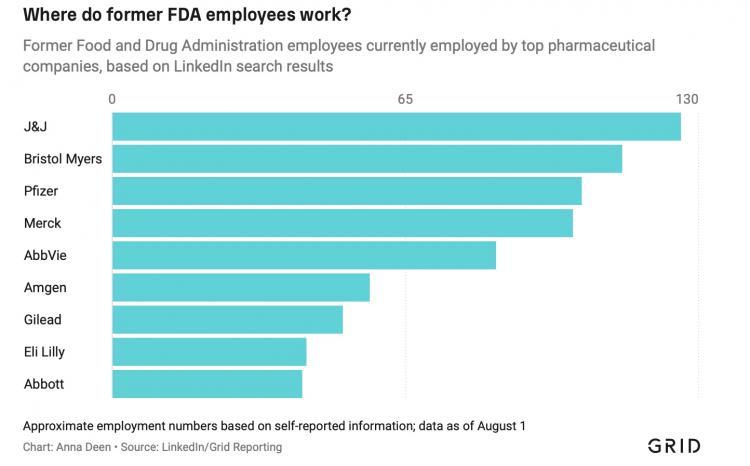
Regulatory oversight agencies say that wherever there is smoke, there is the fire of morality. For decades, they have been warning about the revolving door problem, which has only been well-documented in senior positions and within the Department of Defense. However, hard data on the extent of the problem has been scarce. Grid analysis of LinkedIn personal data shows that at least 2,700 former FDA employees are currently working in the pharmaceutical industry. According to personal profile information, another 1,100 current FDA employees have transferred from the industry to the agency.
Former FDA employees can also be found in many other sectors that the agency regulates: around 1,200 currently work in biotechnology and over 600 work in medical devices. Some notable examples include Trump-era FDA head Scott Gottlieb, who joined Pfizer's board of directors mere weeks after leaving the agency, and current commissioner Robert Califf, who led the FDA during the Obama administration before heading up a health subsidiary of Alphabet Inc. and returning to lead the agency this year. Califf pledged not to work for pharmaceutical or medical device companies within four years of leaving his current position after winning confirmation by the Senate.
The newly established director of the FDA Tobacco Product Center, Brian King, announced on July 26th that Matt Holman has left his position related to tobacco regulatory decisions and had taken a leave of absence prior to July. King expressed his appreciation for Holman's contributions to the center over the years and his unwavering commitment.
Michael Carome, from the Public Citizen organization, which oversees public health in Washington D.C., commented on these figures, stating that they are both interesting and unsurprising. He further added that this number may underestimate the number of employees who transfer to the industry they once regulated, and does not include industry professionals who briefly stay at the regulatory agency before returning to their home industry, another area of concern for ethics experts.
Walter Shaub, the government ethics expert overseeing a government supervision project, stated that "unfortunately, when you see someone moving from a regulatory agency to a regulated industry, the public naturally reacts with a sense of betrayal because it does raise questions about their commitment to the mission of protecting the public." Shaub mentioned that while regulated companies may genuinely want to be responsible for overseeing their people's professional knowledge, "the other thing is that they want to know how the FDA operates inside.
They might be thinking, or people may suspect that they are trying to create an environment where FDA employees know that as long as they do not truly disrupt the regulated industry, there may be a profitable job waiting for them, right? I just think that's human nature," he added.
Carome noted that concerns around revolving doors have fluctuated with the news of measures such as those taken by Holman. During the Trump administration, there was also much concern as cabinet officials and the president himself faced ethics complaints regarding their business ties. Carome added, "There is just really a lack of data on the extent of the problem at the agency staff level.
Dorothy Norris-Tirrell, Chief Learning Officer of the Nonprofit Leadership Alliance, noted that while LinkedIn's dataset includes information on 645 million individuals and 56 million companies, it has limitations. Her own research on career paths in the nonprofit sector utilized LinkedIn data, but she cautioned that the data is self-reported and not consistently updated. However, Norris-Tirrell suggested that the data can be used to identify trends, such as the movement of individuals from the FDA to other industries, their job positions, and tenure at the agency. She also noted that there could be interesting insights gained from segmenting the data. According to LinkedIn's comprehensive records, there are approximately 16,000 people currently employed at the FDA, representing almost 90% of the agency's full-time staff.
In regards to Holman, Philip Morris International stated in a press release issued to Grid that the former FDA official is "dedicated to helping current adult smokers access scientifically validated smoke-free alternatives while also protecting young people.
Holman has been prohibited from communicating with the FDA about Philip Morris and any activity he personally participated in at the agency for a year, according to ethical rules. Holman defended his actions to The New York Times, stating he consulted with the agency's ethics lawyer and believed that if Philip Morris International was transitioning from cigarettes to less harmful tobacco products, he could have entered the industry earlier after working at the FDA for 20 years. (He has not responded to Grid's request for comment.)
However, according to LinkedIn data, many regulatory affairs or drug development employees at pharmaceutical companies under the supervision of the U.S. Food and Drug Administration (FDA), including Merck, Abbott, and Johnson & Johnson, worked at the FDA for over a decade before entering the industry. Diana Zuckerman, President of the National Center for Health Research, said, "The idea that they're avoiding conflicts is nonsense because they're telling companies how to manipulate the system behind the scenes.
Hallman's departure comes as the Biden administration ramps up efforts to regulate the tobacco industry, with plans to mandate reducing nicotine levels in cigarettes to addictiveness and begin phasing out menthol cigarettes. A 2016 law led to a crackdown on e-cigarettes, threatening small e-cigarette shops and resulting in lawsuits against Juul. Last month, the agency ordered the electronic cigarette maker to stop selling its products, despite allowing tobacco giant RJR to sell its own e-cigarettes. The agency's tobacco and food safety plan is currently under external review in light of recent shortages of infant formula and a surge in teenagers using nicotine since 2017.
Stanton Glantz, a long-time critic of the tobacco industry and founder of the Center for Tobacco Control Research and Education at the University of California, San Francisco, expressed that the Tobacco Products Center has made many strange decisions.
Like other tobacco companies, Philip Morris International has shifted towards "non-combustible" tobacco and electronic cigarette devices as part of its efforts to surpass cigarette sales. On March 11th, Holman signed a "modified risk order" from the FDA authorizing the company to use language to market its heated tobacco products, claiming that these products "significantly reduce your exposure to harmful or potentially harmful chemicals.
Glantz stated that he believes the judge is more sympathetic towards Philip Morris when it comes to their language regarding tobacco than public health officials. This comes as the sales of Philip Morris' heated tobacco products have been temporarily suspended in the U.S. as a result of a global patent dispute with competitor RJR, which includes claims of mutual infringement.
Shaub noted that although federal ethics laws require former agency officials to strictly avoid issues they personally handled, these rules do not preclude their colleagues or subordinates from dealing with them. In addition to lacking teeth, the law lacks the ability to protect against punishment beyond the possibility of being fired, which is not much of a threat to those who have already left the institution. While it is not necessarily practical or advisable to prohibit every FDA employee from working in the regulated industry, Shaub called for strengthening rules for those who hold the most critical roles, specifically those who decide to allow profitable products – including tobacco, still America's leading preventable cause of death – which could harm the public.
The Biden administration attempted to address the revolving door phenomenon last year by ordering appointees to pledge not to handle matters related to the agency they oversaw for two years after leaving and to not assist others in lobbying for those positions for a year. Senator Elizabeth Warren of Massachusetts, a Democrat, raised questions about the hiring of officials from the Department of Treasury and the Internal Revenue Service by tax preparation firms and co-sponsored the Anti-Corruption and Public Integrity Act during the Trump administration with the aim of curbing the use of the revolving door by senior officials.
Statement
This article is compiled from third-party information and intended only for industry-related exchange and learning.
This article does not represent the views of 2FIRSTS, and 2FIRSTS cannot confirm the authenticity or accuracy of its content. The translation of this article is solely for industry exchange and research.
Due to limitations in translation ability, the translated article may not fully express the same meaning as the original text. Please refer to the original text for accuracy.
2FIRSTS is in complete alignment with the Chinese government's stance on any domestic, Hong Kong, Macau, Taiwan, and foreign-related statements and positions.
The copyright of compiled information belongs to the original media and author. If infringement occurs, please contact us for deletion.
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.








