
KEY POINTS:
Altria focuses on nicotine pouches: Its subsidiary, Helix, announced the launch of a new product, on! PLUS™, this fall in three U.S. states (North Carolina, Texas, and Florida).
FDA approval is still pending: Currently, the FDA has only approved Philip Morris International's (PMI) ZYN for sale in the U.S.; on! PLUS™ has not yet been approved.
Titans are racing to the market: British American Tobacco (BAT) plans to pilot its disposable synthetic nicotine e-cigarette, Vuse One, in the U.S. in late September, a product also lacking FDA approval.
Significant market overlap: The launch of on! PLUS™ and Vuse One is highly similar in both timing and initial markets, such as Florida and Georgia.
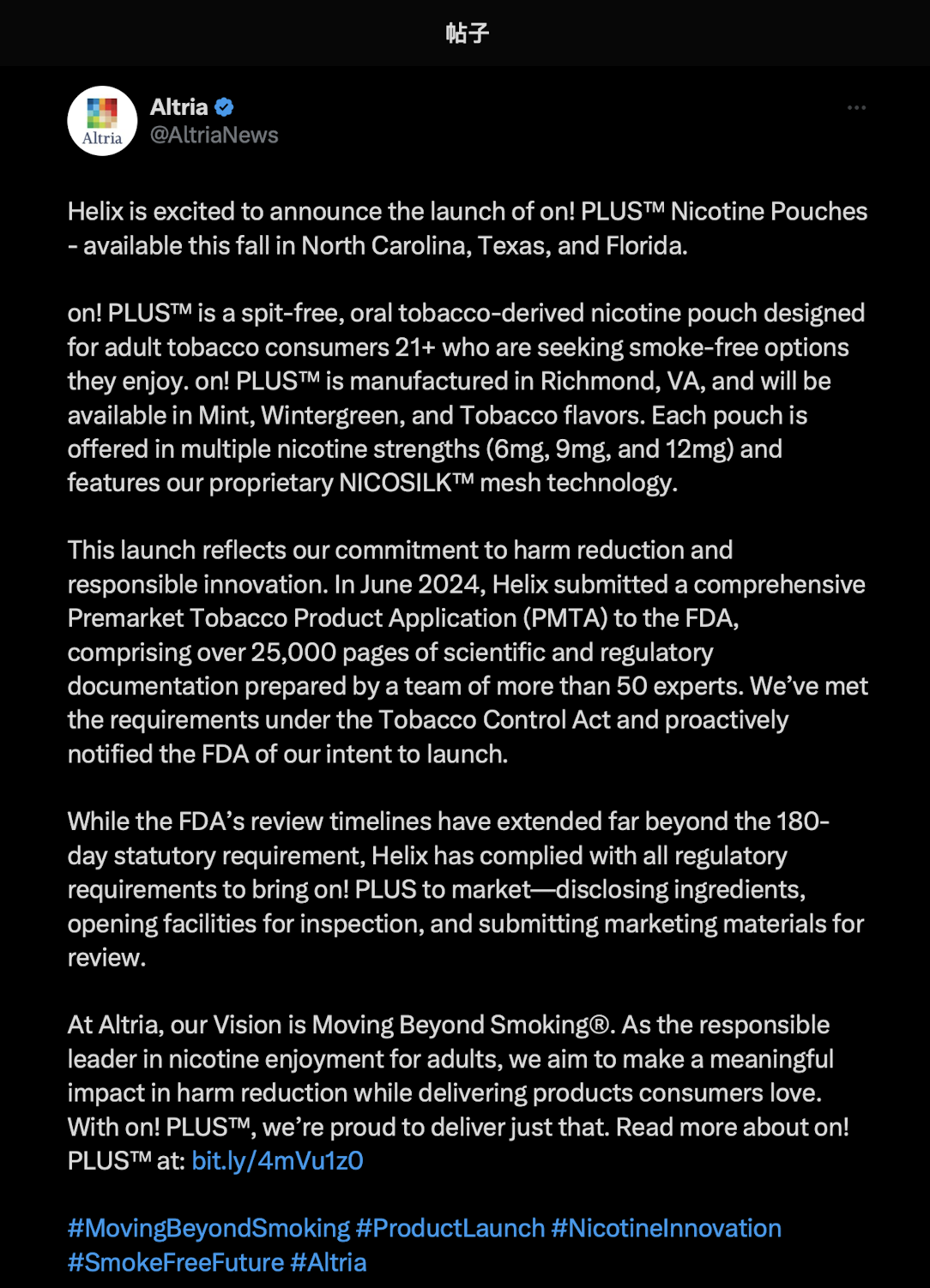
On August 22, 2Firsts noticed that Altria officially announced on its X (formerly Twitter) account that its subsidiary, Helix, will officially launch a new nicotine pouch product, on! PLUS™, this fall in North Carolina, Texas, and Florida.
According to the announcement, on! PLUS™ is a spit-free, tobacco-derived oral nicotine pouch designed for adult consumers aged 21 and over, aiming to provide a more convenient smokeless alternative. Produced at its Richmond, Virginia factory, the product will initially be available in Mint, Wintergreen, and Tobacco flavors, with nicotine strengths of 6mg, 9mg, and 12mg. It features the unique NICOSILK™ mesh technology.

Altria stated that Helix submitted a premarket tobacco product application (PMTA) to the FDA in June 2024, which spanned over 25,000 pages. Written by more than 50 experts, the application covered scientific and compliance research in accordance with the requirements of the Tobacco Control Act. Helix has also proactively informed the FDA of its launch plans.
Altria added that despite the FDA's review period far exceeding the statutory 180-day limit, Helix has strictly followed all compliance procedures, including ingredient disclosure, factory inspections, and submission of marketing materials.

Below is the content posted by Altria on its official X social media account:
Currently, the FDA has only approved Philip Morris International's (PMI) nicotine pouch brand, ZYN, for legal sale in the U.S. There is no public information about the approval of on! PLUS™.
Notably, just one day before Altria's announcement (August 21), Reuters reported that British American Tobacco (BAT) plans to pilot its disposable synthetic nicotine e-cigarette, Vuse One, in the U.S. It is expected to launch in late September in South Carolina, Florida, and Georgia, a product that also has not received FDA approval.
It is worth noting that on! PLUS™ and Vuse One not only have very similar launch timelines (one in the fall, the other in late September), but their initial markets also have significant overlap, as both plan to enter Florida and Georgia.







