
Update:
Following publication, the FDA issued an official press release confirming that six on! PLUS nicotine pouch products had received Marketing Granted Orders (MGO) through the PMTA pathway. The announcement states that the authorizations were completed under the agency’s nicotine pouch review pilot program and were finalized in record time. A screenshot of the FDA press release page is included below.
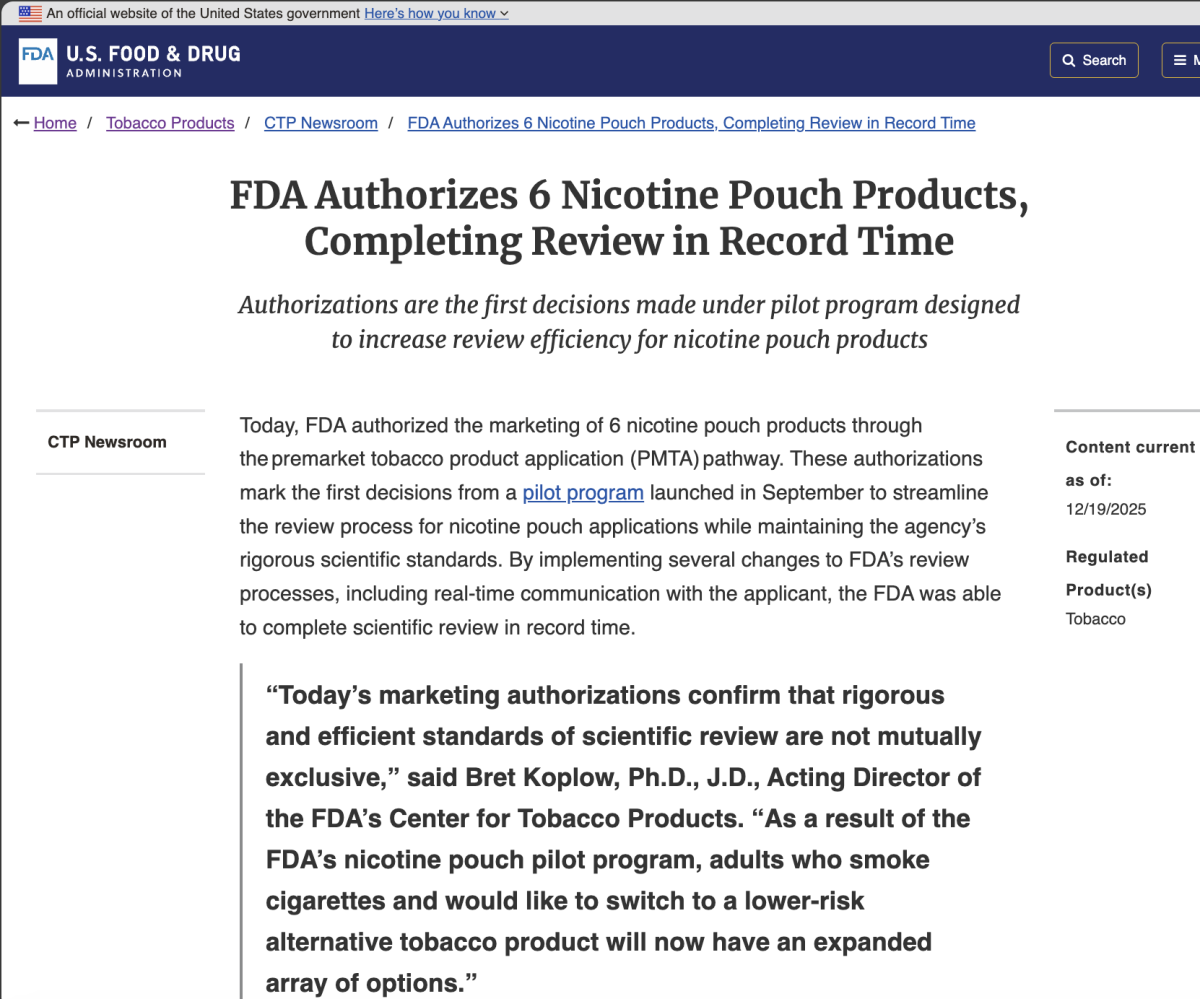
Key Points:
- The FDA has added on! Plus nicotine pouches to its list of PMTA-authorized products.
- on! Plus is the second nicotine pouch brand to receive a Marketing Granted Order (MGO), after ZYN.
- The authorization was disclosed through an update to the FDA’s website; no separate press release had been issued as of publication.
2Firsts ,December 19, 2025,The U.S. Food and Drug Administration (FDA) has updated its online list of nicotine pouch products authorized by the agency, disclosing that on! Plus nicotine pouches have received Marketing Granted Orders (MGO) via the Premarket Tobacco Product Application (PMTA) pathway—allowing the products to be legally marketed in the United States.

The update makes on! Plus the second nicotine pouch brand authorized via PMTA, alongside Swedish Match’s ZYN.
As of publication, the FDA had not issued a separate press release on the decision, disclosing the authorization instead through an update to its list of authorized nicotine pouch products.

What’s Authorized (FDA List)
According to the FDA’s “Nicotine Pouch Products Authorized by the FDA” page, the authorized on! Plus SKUs include:
on! PLUS nicotine pouches 6 mg Mint
on! PLUS nicotine pouches 9 mg Mint
on! PLUS nicotine pouches 6 mg Tobacco
on! PLUS nicotine pouches 9 mg Tobacco
on! PLUS nicotine pouches 6 mg Wintergreen
on! PLUS nicotine pouches 9 mg Wintergreen
These additions appear on the same FDA-authorized list that includes multiple ZYN nicotine pouch products previously granted marketing orders through PMTA.
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.




