Disclaimer:
1. This article solely addresses issues of e-cigarette regulation and commerce, intended for readers within the global next-generation tobacco industry.
2. The content does not analyze or comment on political or diplomatic matters. Nothing herein may be cited for political or diplomatic purposes.
3. Opinions and remarks from individuals referenced in this article are conveyed objectively and do not reflect the views of 2Firsts. No judgment is made on their perspectives in other domains.
4. Statements related to politics and diplomacy are based on official declarations.
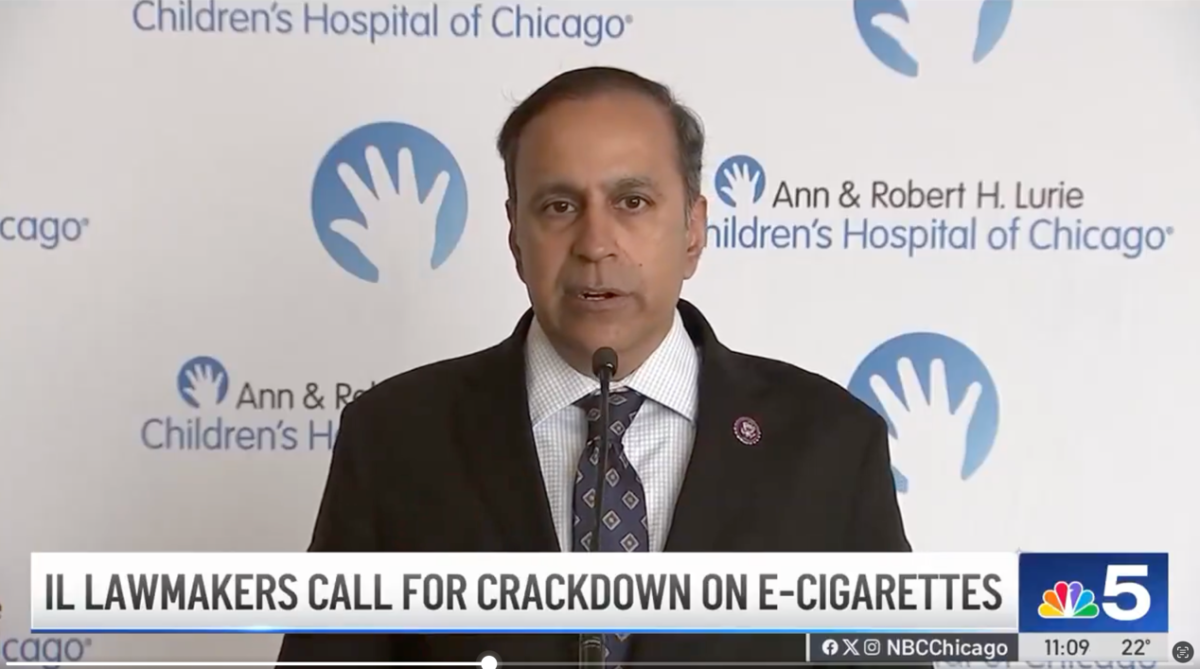
[Reported by 2Firsts] According to an announcement published on the U.S. House of Representatives website, on December 2, 2024, Chicago time, Raja Krishnamoorthi, the Ranking Member of the Select Committee on the Strategic Competition Between the United States and the Chinese Communist Party, joined local officials in discussing efforts to combat youth vaping. During the meeting, he announced a new investigation into Chinese illicit vape manufacturers. Key points from the announcement include:
- Approximately 95% of global vaping products are manufactured in China, including popular brands such as Elf Bar. Despite this, the U.S. Food and Drug Administration (FDA) has authorized only 34 tobacco-flavored e-cigarette products for sale nationwide. (Original text reads "tobacco-flavored e-cigarette products". In fact, on June 21, 2024, the FDA approved four menthol-flavored e-cigarette products from NJOY for the first time. Note by 2Firsts.)
- A U.S. county sheriff stated, "Like the opioid crisis, the unrelenting flood of toxic vaping devices, flavored like candy and marketed to appeal to youth, is a public health crisis that demands federal resources and solutions."
- Krishnamoorthi urged the FDA to act decisively to remove all illicit vaping products from shelves in Cook County, across Illinois, and throughout the United States.
- Throughout his tenure, Krishnamoorthi has advocated for stricter regulations on e-cigarettes and vaping products, including his 2019 investigation into JUUL, which uncovered targeted advertising aimed at youth. He also co-chairs the bipartisan Congressional Caucus to End the Youth Vaping Epidemic.
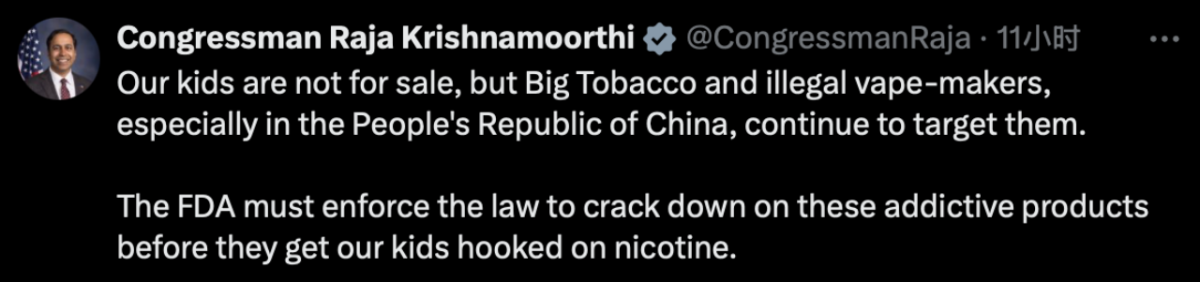
On the same day, Congressman Raja Krishnamoorthi took to social media platform X to criticize Big Tobacco and illicit e-cigarette manufacturers for targeting children. He also urged the FDA to take swift action to address the issue.
Youth Vaping and Illicit E-Cigarettes Under Scrutiny
In recent months, U.S. attention on youth vaping and illicit e-cigarette concerns has intensified.
In October 2024, the FDA and Customs and Border Protection (CBP) seized approximately 3 million unauthorized e-cigarette products with an estimated retail value of $76 million—the largest financial seizure of its kind to date.
That same month, 2Firsts corresponded with the FDA regarding regulatory policies. In its response, the FDA emphasized that only 34 e-cigarette products are currently authorized for legal sale and provided a comprehensive list of approved products. View the full list here.
In November, a U.S. research organization reported a 500% increase in disposable vape sales between 2019 and 2023, making them the most popular device among youth. As of March 2024, the 34 FDA-authorized products accounted for just 13.7% of total retail sales in the U.S.
International Cooperation on E-Cigarette Regulation Gains Diplomatic Traction
Collaboration on e-cigarette regulation has expanded into the realm of diplomacy.
In June 2024, during a meeting between the Chinese and Australian prime ministers, a joint statement was released emphasizing cooperation on border enforcement, including the fight against illicit tobacco and e-cigarette smuggling. This marked the first time such issues were explicitly mentioned in a bilateral statement.
Leading up to this meeting, there were multiple high-level engagements between regulatory and enforcement agencies from both nations. For example, on May 6, 2024, Chinese officials(STMA) met with Australia's Deputy Commissioner of Border Enforcement to discuss collaborative measures.
2Firsts will continue to track these developments and provide in-depth coverage on the evolving landscape of global e-cigarette regulation.
We welcome tips and comments. Please contact us at info@2firsts.com, or connect with 2Firsts CEO Alan Zhao on LinkedIn (here).
Cover image: NBC Chicago's News Coverage on the Matter | Image Source: NBC Chicago







