
Statement:
This article is a repost from Reuters, with copyrights belonging to the original author and publishing organization.
Reproduction is solely for the purpose of information dissemination and industry reference, and does not constitute any investment, legal, or business advice. It also does not represent the views, position, or endorsement of this platform.
This platform makes no guarantee of the accuracy, completeness, or timeliness of the original content.
Key Takeaways:
Regulatory Action: The United States Postal Service (USPS) has banned non-compliant e-cigarette distributor Demand Vape from using its services, revoking its mailing exemptions due to transporting products without FDA authorization and violating flavor bans; USPS has also provided a list of other exempted e-cigarette companies to the New York City Law Department, potentially further restricting industry transportation channels.
Industry Impact: The non-compliant e-cigarette market is sizable (estimated to reach around $8.05 billion by 2024 in the US and UK), but relies on limited transportation channels, with USPS's actions potentially exacerbating operational costs and survival pressures, favoring traditional tobacco giants.
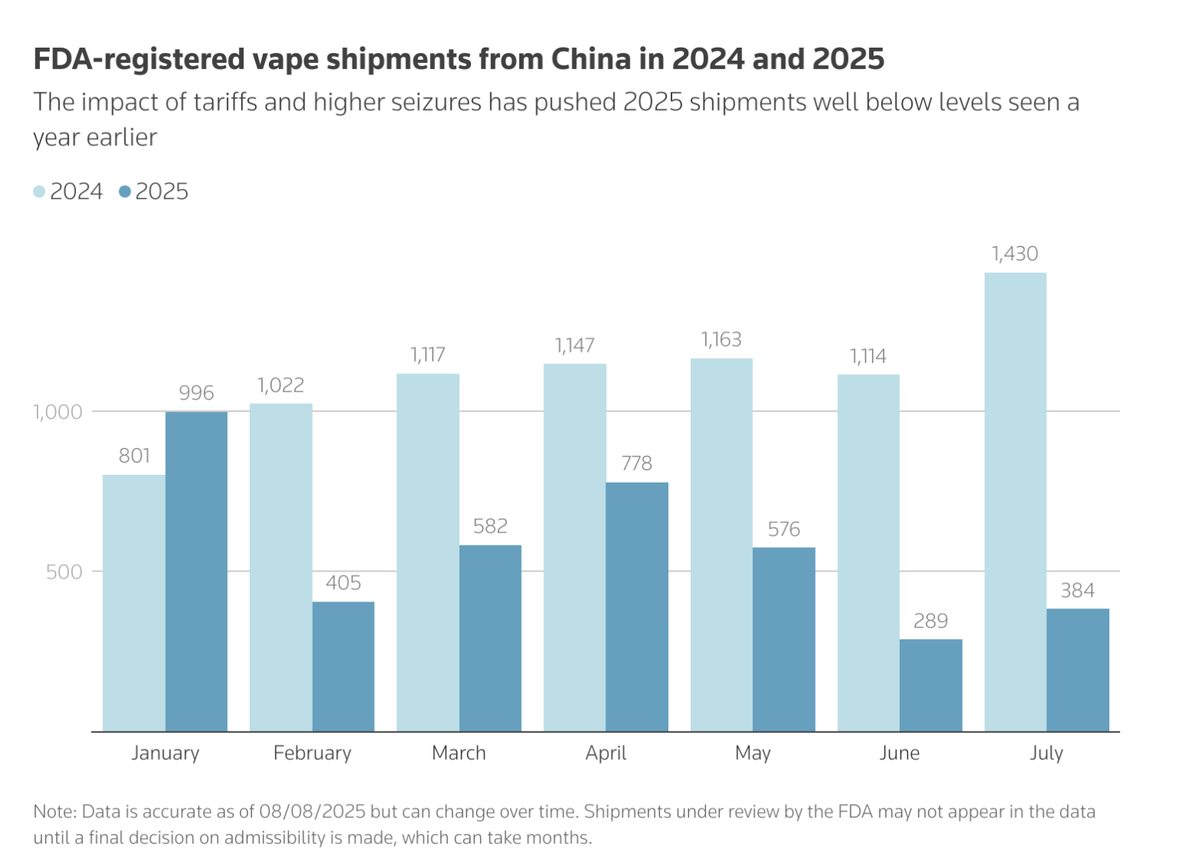
Background and Current Situation: Only 39 types of e-cigarettes in the US have FDA authorization, while non-compliant products are widely circulated; since 2025, increased import tariffs, port seizures, and FDA crackdowns on middlemen have increased industry pressures, leading to some stores having empty shelves.
Company Reactions: Demand Vape claims to be compliant with the law, questioning the revocation decision and stating that the industry is in a "regulatory gray area"; industry associations state that USPS's actions will further harm American e-cigarette companies.
According to a report from Reuters on August 11, a review of documents by Reuters has revealed that the United States Postal Service (USPS) has begun cracking down on non-compliant e-cigarette distributors using its services for commercial transportation.
These previously undisclosed letters show that USPS has sent a letter to the main distributor, Demand Vape, based in New York, prohibiting them from using USPS services. The reason is that the New York City Law Department, representing the city government and officials in legal matters, provided evidence showing that the company's transportation practices are in violation of the law.
Reuters reported that the actions taken by USPS could benefit tobacco companies like Altria and British American Tobacco. These companies have been struggling for years against the proliferation of non-compliant e-cigarettes mainly coming from China.
Noncompliant e-cigarettes that have not received authorization from the U.S. Food and Drug Administration (FDA) must obtain this authorization in order to legally sell in the United States, the world's largest market for smoking alternatives.
The USPS sent a letter to Demand Vape on July 15 stating that they have revoked their mailing privileges as of July, after receiving evidence that the company's shipment of e-cigarettes lacked FDA authorization and violated local flavor bans.
Demand Vape has stated that it is complying with relevant laws and is challenging the decision to revoke its authorization. The company also said that the industry is in a "regulatory gray area", with only a few products authorized by the FDA not being able to meet consumer demand.
The company stated in a press release:
"We oppose any characterization of Demand Vape as an opaque, illegal, or disreputable company."
The USPS did not respond to a request for comment.
Limited exemptions
Currently, the US FDA has only approved 39 types of e-cigarette products. However, unauthorized e-cigarette devices continue to circulate widely due to difficulties in regulation.
According to a law passed in 2021, USPS is restricted from directly mailing e-cigarettes to consumers, conducting international mailings of e-cigarettes, and in most other situations from mailing e-cigarettes.
Limited exemptions include domestic transportation between businesses, which requires a "mailing exemption," and the products being transported must comply with relevant laws.
Several major courier companies, including FedEx, have refused to transport e-cigarettes. DHL Freight Airlines only provide services for the transportation of e-cigarettes for businesses with prior approval.
Eric Proshansky, Deputy Director of the Litigation Department in New York City, told Reuters that USPS has provided the City's Law Department with a list of other e-cigarette companies that have been granted mailing exemptions. The department will evaluate whether it should raise concerns about these companies based on legal requirements.
This could potentially further reduce the number of transporters available to the non-compliant e-cigarette industry. Other options, such as using small transporters or handling freight directly, are often more costly.
The pressure is increasing day by day
According to British American Tobacco, the estimated value of the non-compliant e-cigarette market in 2024 is approximately £6 billion (around $8.1 billion USD). However, the market is facing increasing pressure. In 2025, the United States' import tariffs and port seizure measures have decreased the import volume of non-compliant e-cigarettes.
As part of the May enforcement action, the FDA also sent letters to 24 American middlemen, including key distributors in the non-compliant e-cigarette market.
Tony Abboud, executive director of the Vapor Technology Association representing companies including Demand Vape, stated that this has resulted in empty shelves at e-cigarette shops.
He said that the revocation of USPS exemptions will further harm American e-cigarette companies.
According to the lawsuit filed against Demand Vape by the city of New York in 2024, as one of the largest e-cigarette distributors in the United States, Demand Vape supplies approximately 5,000 retailers across 49 states.
According to another letter reviewed by Reuters, the city prosecutor provided evidence to USPS, including copies of invoices showing Demand Vape selling unauthorized e-cigarettes, some of which were brands specifically marked by the FDA as being illegal for sale.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








