
On January 3rd, the U.S. Food and Drug Administration (FDA) updated its import alerts and released a press release on its official website titled "FDA Updates Import Alerts to Reinforce that All Unauthorized E-Cigarettes May Be Detained Without Physical Examination.

The news release states that the U.S. Food and Drug Administration (FDA) has announced updates to two import alerts, namely 98-07 and 98-06. These updates will distinguish imported e-cigarette products from all other tobacco products, providing clearer guidance for FDA frontline staff, federal partners, and a broader trade community including importers, customs brokers, and declarants.
The FDA stated that if a product or company is suspected of violating FDA regulations, the FDA may place the product or company on an import alert list, and subsequently detain the products without the need for testing or physical examination.
The press release also mentioned that the FDA's update on Import Alert 98-07 emphasizes that any unauthorized e-cigarette products imported into the United States may be detained without physical examination and refused entry by the FDA.
The update to the import alert from 1998 to 2006 now focuses on imported tobacco products other than e-cigarettes. This import alert covers unauthorized tobacco products such as smokeless tobacco and nicotine pouches, and has recently added nicotine pouch products from brands like NOIS, LYFT, and SKRUF. These tobacco products may also be detained by import officials, without physical examination, and denied entry by the FDA.
It is worth noting that the FDA also mentioned that an application under review does not create a legal safe harbor for the distribution or sale of unauthorized products.
Below is the full text of the press release (content translated by 2Firsts using AI, subject to FDA original text):
On January 3rd, the US FDA announced updates to two import alerts, numbered 98-07 and 98-06. These updates will distinguish imported e-cigarette products from all other tobacco products and provide clearer guidance for FDA frontline staff, federal partners, and a broader trade community including importers, customs brokers, declarants, and others.
If a product or company appears to violate FDA laws and regulations, the FDA can place the product or company on an Import Alert list, and subsequently detain future shipments of the product without the need for testing or physical inspection.
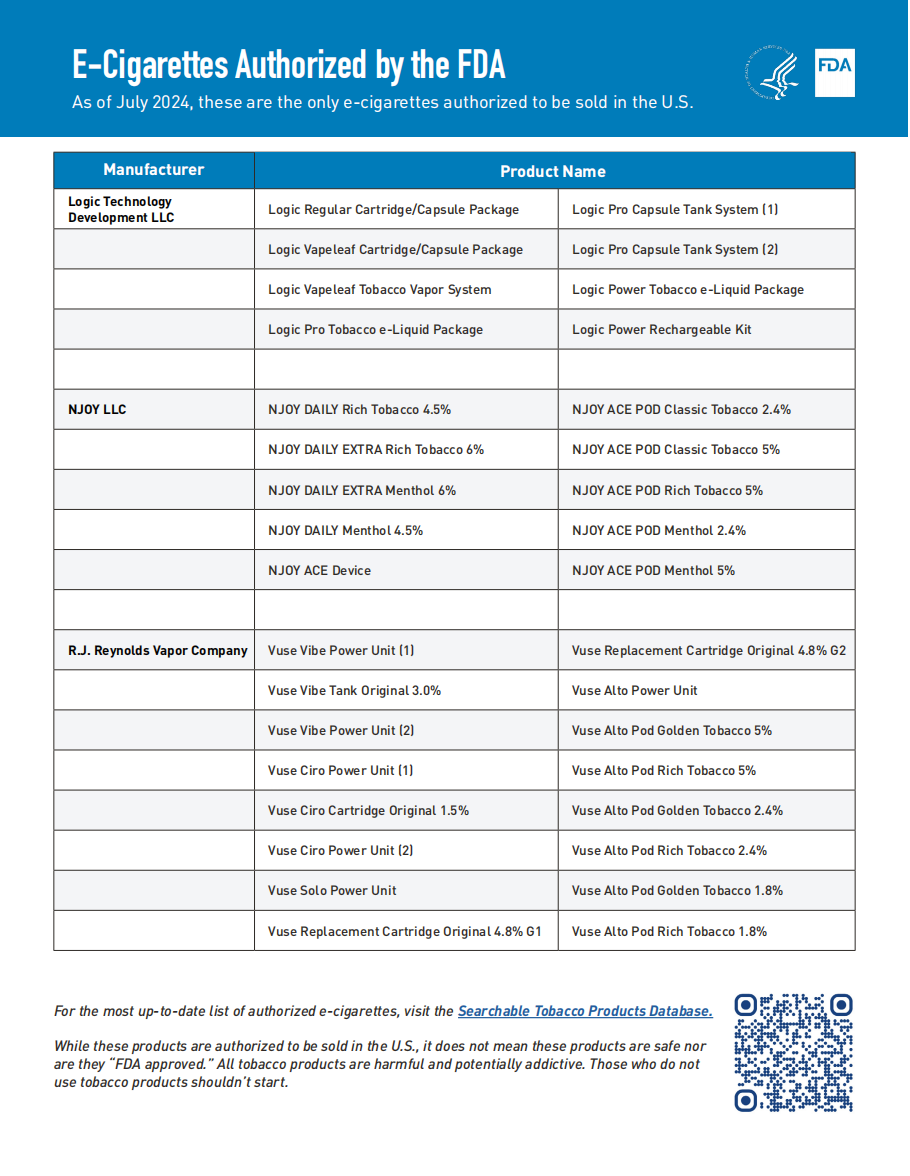
The update on Import Alert 98-07 emphasizes that any unauthorized e-cigarette products imported into the United States may be detained without physical examination and refused entry by the FDA. This has been and will continue to be the FDA's policy; however, these updates will help clarify the requirements for those affected by the alert and those responsible for enforcing it. Specifically, Import Alert 98-07 has been simplified to include a link to the FDA's searchable tobacco product database and a downloadable list of authorized e-cigarette products eligible for legal importation into the United States. The FDA's website further elaborates on the agency's enforcement priorities for tobacco products, including a few specific situations in which the FDA does not currently intend to take enforcement actions against unauthorized e-cigarette products.
The revised e-cigarette import warning also reinforces a basic legal requirement that all new tobacco products, including e-cigarettes, must be authorized by the FDA in order to be legally sold in the United States. The FDA does not have a broad enforcement discretion policy for unauthorized e-cigarette products. It is worth noting that an application that is currently under review does not create a legal safe harbor for distributing or selling unauthorized products.
The FDA has also updated Import Alert 98-06 to focus on imported tobacco products other than e-cigarettes. This import alert covers unauthorized tobacco products in categories such as smokeless tobacco and nicotine pouches, with recent additions including brands such as NOIS, LYFT, and SKRUF. These tobacco products may also be detained by import officials, denied entry by the FDA without physical inspection.
In addition to issuing import alerts for accurately declaring products, the agency continues to collaborate with federal partners to address falsely declared products. For example, in October 2024, the FDA and U.S. Customs and Border Protection announced the successful completion of a joint operation that legally seized approximately 3 million unauthorized e-cigarette products with an estimated retail value of $76 million. Such actions reflect the enhanced cooperation and coordination among federal agencies as part of a special task force to curb the distribution and sale of illegal e-cigarettes.
Next, 2Firsts will conduct an in-depth analysis and detailed interpretation of this policy. Stay tuned for updates.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








