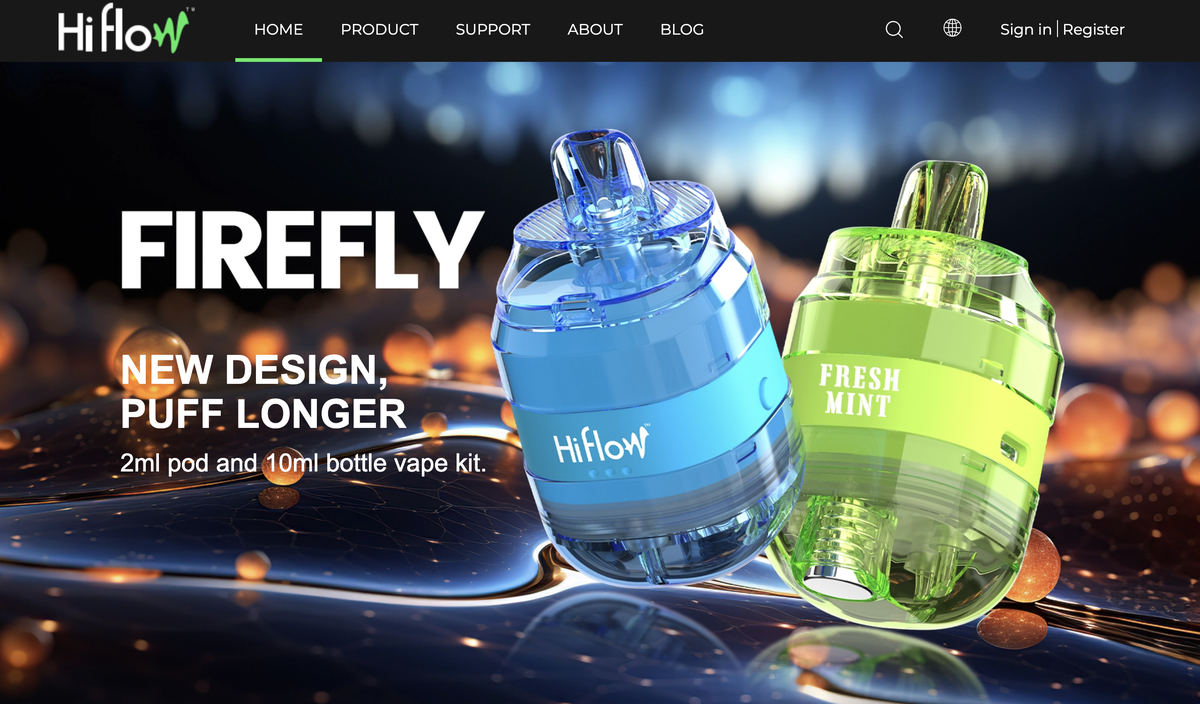
At a recent tobacco exhibition in Dortmund, 2Firsts discovered a new trend in "2+10" products, with one standout being the Hiflow FIREFLY e-cigarette designed in the shape of a wine barrel. The product has now been launched on the official website of the Hiflow brand.

More specifically, the main product features of Hiflow FIREFLY include:
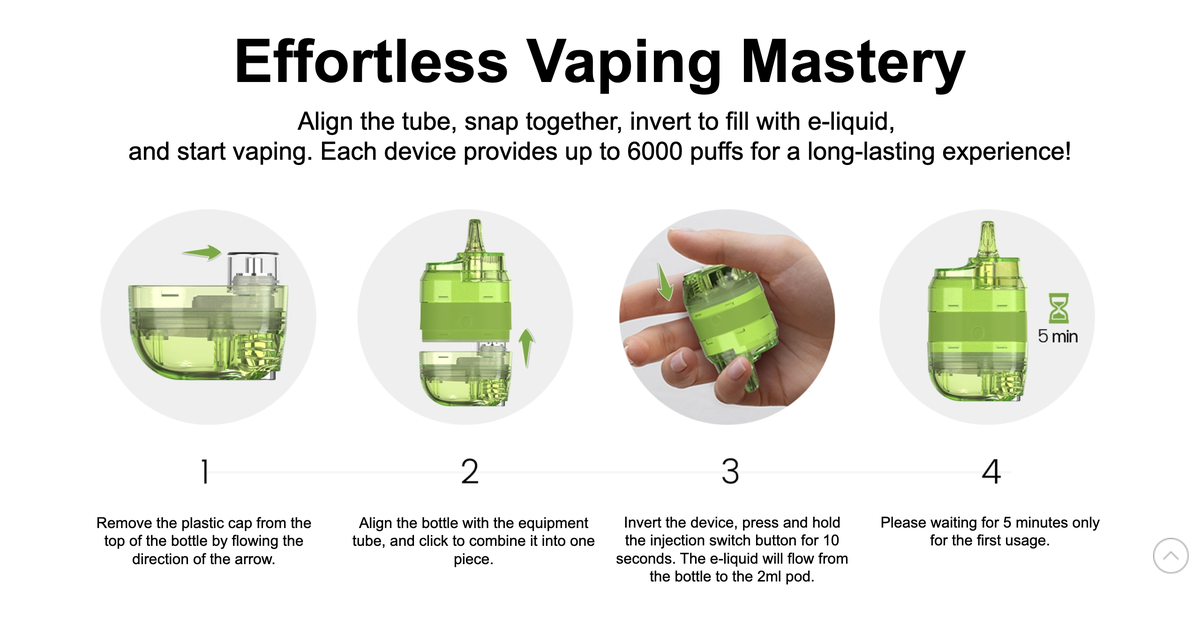
E-liquid capacity: 2ml pod + 10ml pre-filled e-liquid Nicotine concentration: 2% Number of puffs: 6000 puffs Battery capacity: 450mAh Charging performance: Supports Type-C fast charging Flavors: 15 flavors Features: Refillable e-liquid, mesh heating, child lock Compliance: TPD standards
In terms of appearance, the Hiflow FIREFLY resembles a small "barrel" style, with a rounded shape that is less common in the current e-cigarette market. Additionally, unlike other disposable e-cigarette products that are marketed as "2+10", the Hiflow FIREFLY features a pre-filled and refillable design, allowing for a longer usage time.
When the e-liquid in the top pod of the product is running low, simply remove the plastic cap of the pre-filled e-liquid bottle, align and attach it to the device's tube, then invert the device, hold down the injection switch button for 10 seconds, and the pre-filled e-liquid will flow from the pre-filled e-liquid bottle into the 2ml pod. After supplementing the e-liquid, wait 5 minutes before using the product.
However, according to a survey by 2Firsts, Hiflow FIREFLY is currently only found on the brand's official website and the Made-in-China website, and has not yet appeared on the websites of major overseas distributors.
Firsts will continue to monitor the technological innovations and market performance of "2+10" products in the e-cigarette industry, providing accurate and authoritative information to the public.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








