
Special statement:
1. This article is for internal industry communication only and does not make any recommendations for brands or products.
2. The images shown in this article are only used to describe facts and are not intended as advertisements for any products.
3. This article is prohibited for minors to access.
Based on a systematic observation of global e-cigarette product trends conducted by 2Firsts from August 25-29, 2025, the UK market is sending new signals in terms of regulatory announcements and product innovation. This issue focuses on the trends in the UK market and the movements of well-known brands.
Recent Developments in the UK: Continuous Innovation in Pod Systems
According to a statistical analysis by 2Firsts of public data from the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK, during the period of August 18-24, MHRA only listed 193 e-cigarette SKUs, a significant decrease of about 86.5% compared to the previous week (1156 SKUs).
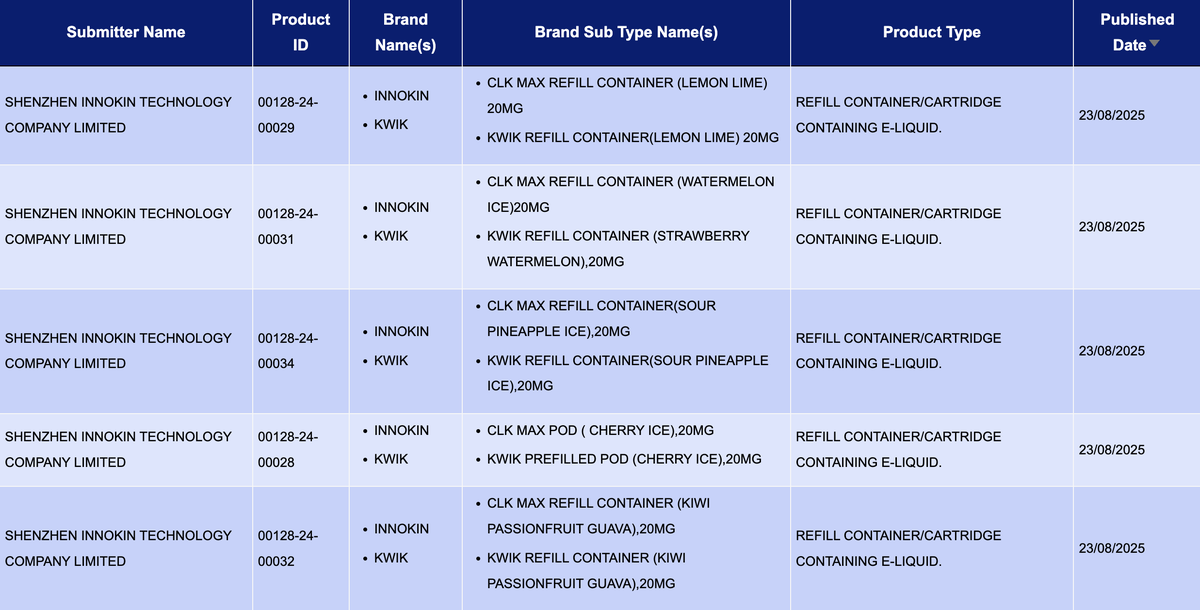
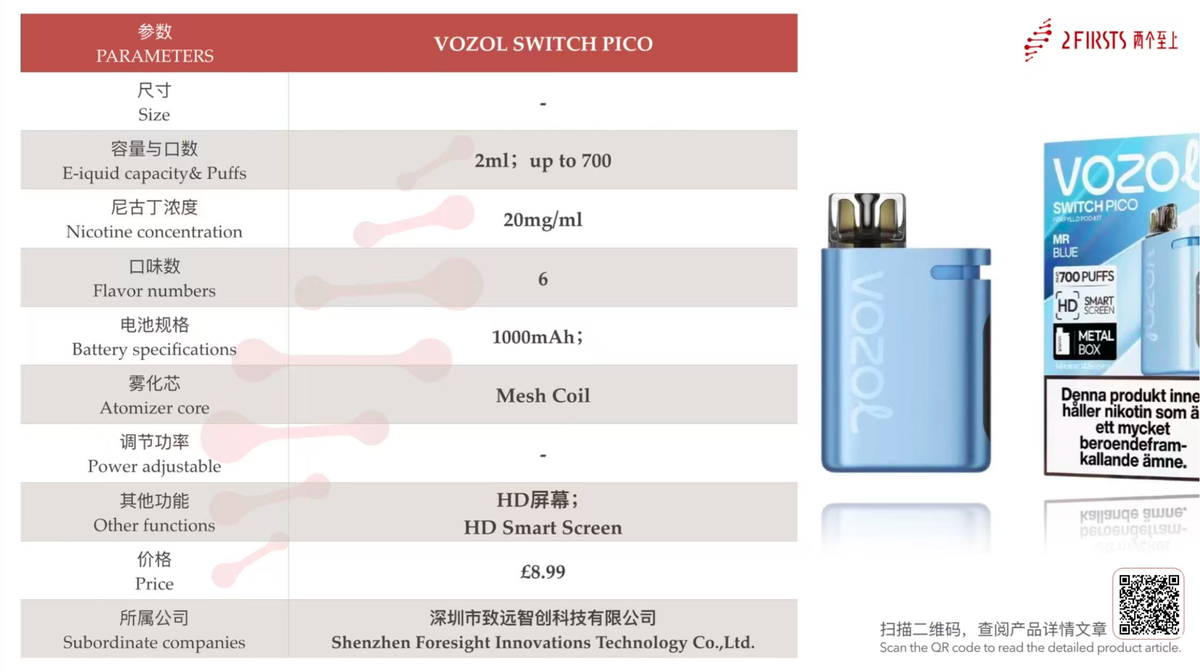
At the same time, brands like VOZOL and SKE have launched new pod system devices in the United Kingdom, showcasing their strategic positioning in the pod system product market and their commitment to exploring smoke-free experiences.

Differentiation breakthrough: ELFBAR, OXBAR accelerates personalized and functional layout.
ELFBAR and OXBAR have recently stepped up their product differentiation efforts. ELFBAR has released the limited edition ELFA MASTER Stein, which not only continues the series' compatibility but also offers independent numbering and custom engraving services, highlighting its personalized positioning. On the other hand, OXBAR has introduced TRI-FUSION, a new design featuring "three compartments and three controls." Users can adjust sweetness, nicotine concentration, and cooling sensation separately, claiming to be able to create up to 100 different flavor experiences.


As a leading global NGP media and think tank, 2Firsts is committed to providing the latest product and technology information and insights to practitioners worldwide in various categories such as e-cigarettes, heated tobacco products, and modern oral products. It aims to drive technological change and innovation in NGP products worldwide, ultimately bringing safer products and lifestyles to tobacco consumers globally.
With its information sources covering the Chinese supply chain and global markets, 2Firsts' product reports have become one of the most influential platforms for new product and technology releases worldwide. Contact 2Firsts for more information.
1.Provide clues to new products and technologies.
2.Provide comments on products and technology.
3.Seek media coverage for your product.
4.Gather information on the product's distribution channels.
Contact information:
1. Email: info@2firsts.com
2. Contact 2Firsts CEO, Alan Zhao, on LinkedIn.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








