Special Statements:
1.This article is for internal industry communication only and does not make any recommendations for brands or products.
2.The images displayed in this article are only used to describe facts and are not intended as advertisements for any products.
3.This article is prohibited for minors to access.
Key Points:
·In the first week of June, the UK Medicines and Healthcare products Regulatory Agency (MHRA) announced that there were a total of 194 new SKUs of refill containers/pods containing e-liquid in their e-cigarette product notification database.
·The newly released e-liquid products offer a wide variety of flavors, with fruit, mixed fruit, and iced flavors being predominant. All products strictly comply with the regulatory limit of 20mg/ml for nicotine content.
·Innokin and Double Drip brands are both publicly showcased simultaneously.
·VAPORLAX announces two open system products and standalone components.
【By 2Firsts】On June 1st, Britain officially implemented a nationwide ban on the sale of disposable e-cigarettes that cannot be refilled with e-liquid or have non-rechargeable batteries. In the first week of June, two individuals conducting inquiries on the Medicines and Healthcare products Regulatory Agency (MHRA) e-cigarette product announcement database discovered that several e-cigarette brands, including RELX, FUMOT, and HAYATI, have announced new SKUs. After completing the announcement process with the MHRA, these new SKUs indicate that they have received market approval and will soon be officially entering the British market for sale.
Disposable E-Cigarettes Delisted; 194 Pod Products Newly Registered
2Firsts has compiled and organized the public information from the Medicines and Healthcare products Regulatory Agency (MHRA) e-cigarette product alert database for the first week of June in the United Kingdom. Below are the details of the alerts.
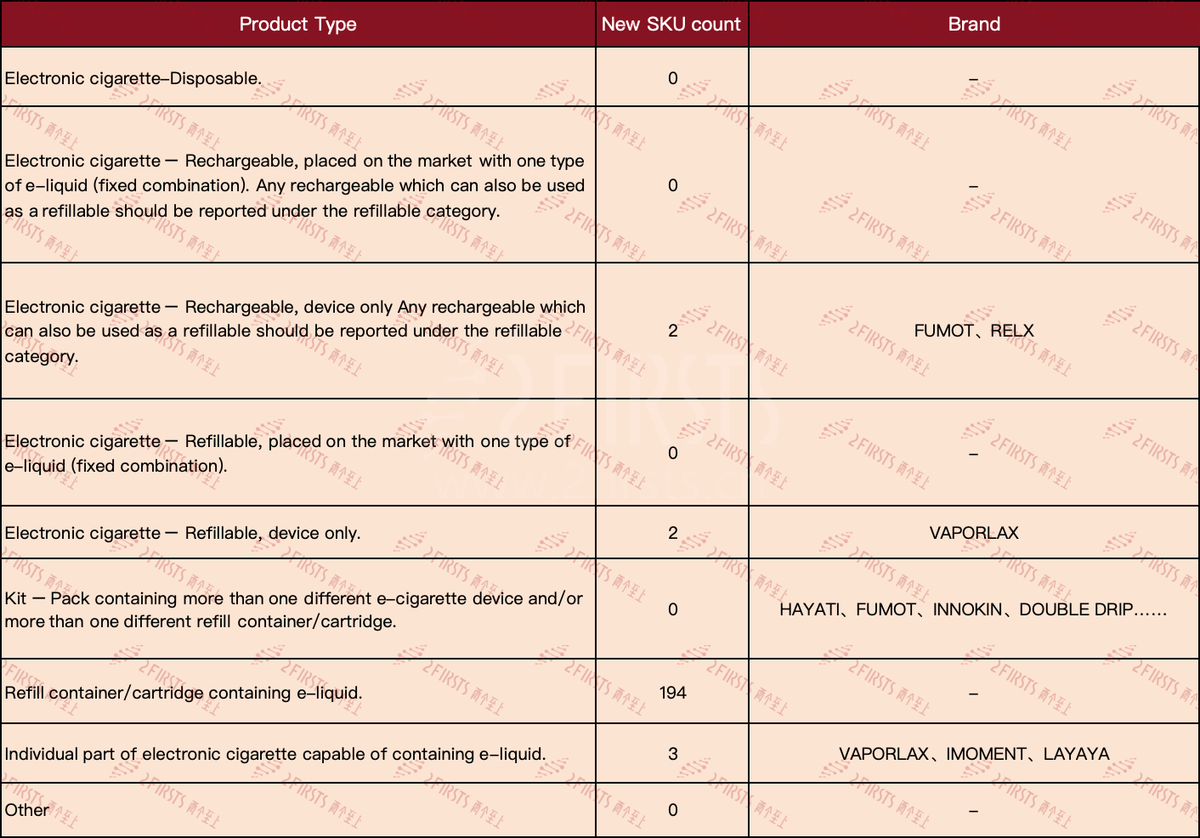
According to the data shown in the table for the first week of June, it is apparent that there were no new SKUs for "Electronic cigarette—Disposable" products. Additionally, 2Firsts also noticed that there were no longer any public announcements for e-cigarette products in this category, indicating that manufacturers are no longer declaring new disposable products that cannot be filled with e-liquid or have non-rechargeable batteries for the UK market.
·The number of new SKUs for the "Electronic cigarette—Rechargeable, device only" category is 2, involving the brands FUMOT and RELX.
·The new SKU "Electronic cigarette—Refillable, device only" has a total of 3 brands, namely VAPORLAX, IMOMENT, and LAYAYA.
·The most notable is the "Refill container/cartridge containing e-liquid," with a new SKU count as high as 194.
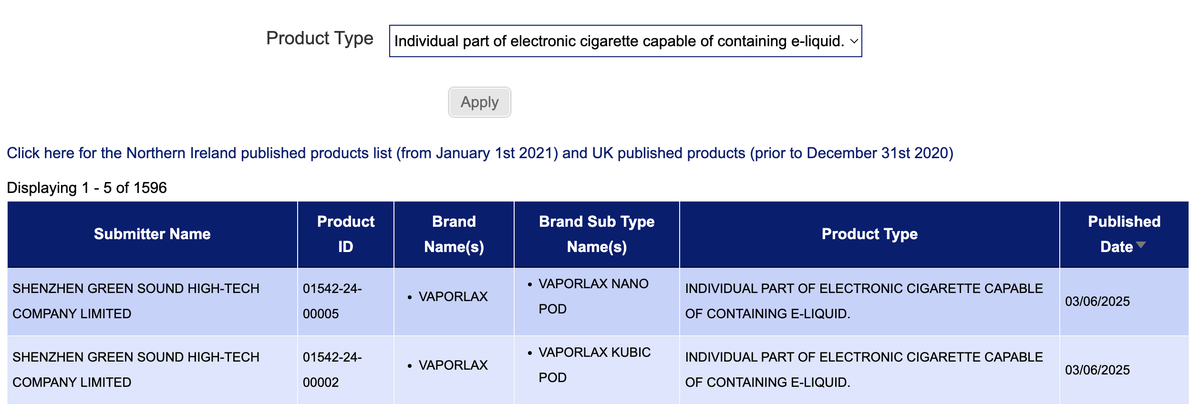
·New SKUs available for individual components of electronic cigarettes that can contain e-liquid, branded as VAPORLAX.
HAYATI, FUMOT, INNOKIN Roll Out Multiple 20mg/ml Pod Products
In the first week of June, the UK Medicines and Healthcare products Regulatory Agency (MHRA) updated a total of 194 SKUs in the e-cigarette product notification database under the category of "Refill container/cartridge containing e-liquid." This includes brands such as HAYATI, FUMOT, INNOKIN, DOUBLE DRIP, and others.
2Firsts has collected and compiled detailed information on the "Refill container/cartridge containing e-liquid" category for the first week of June. The specifics are shown in the following chart:

The e-cigarette brand HAYATI posted 60 "Refill container/cartridge containing e-liquid" SKUs on the UK Medicines and Healthcare products Regulatory Agency (MHRA) e-cigarette tracking system in the first week of June. 2Firsts collected and analyzed the information, revealing the following product characteristics:
·All flavors are clearly labeled with a nicotine content of 20mg/ml.
·The announcement focuses mainly on fruit and mixed fruit flavors.
·Different series or capacities of e-liquid products with the same flavor have been launched to cater to different device models.
During the first week of June, FUMOT made 32 "Refill container/cartridge containing e-liquid" products available in the UK Medicines and Healthcare products Regulatory Agency (MHRA) e-cigarette query system. 2Firsts gathered and analyzed the public information, identifying the following product characteristics:
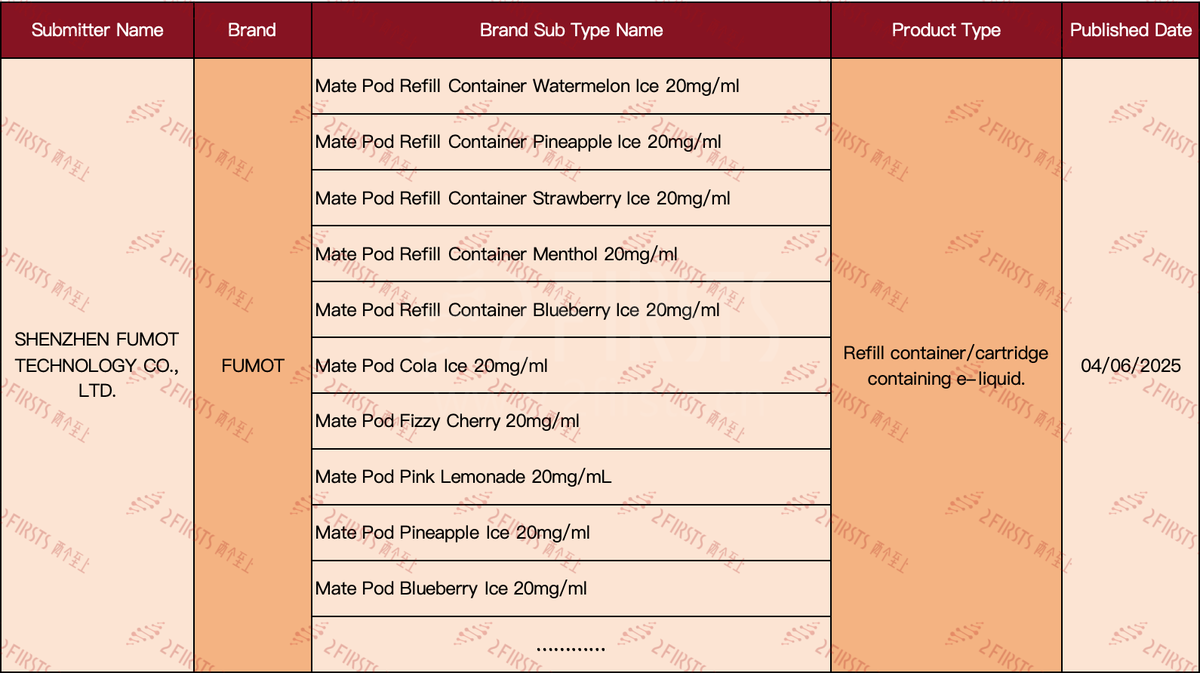
·FUMOT has declared a variety of flavors of e-liquid, all prefaced with "Mate Pod Refill Container," indicating they are designed for its Mate Pod series of refillable pods or e-liquids.
·All flavors are clearly marked with a nicotine content of 20mg/ml.
·A considerable portion of the flavor names contain the word "Ice," emphasizing a cool or chilled sensation.
In the first week of June, INNOKIN listed 10 "Refill container/cartridge containing e-liquid" SKUs on the UK Medicines and Healthcare products Regulatory Agency (MHRA) e-cigarette database. 2Firsts collected and analyzed the information and found the following product characteristics:
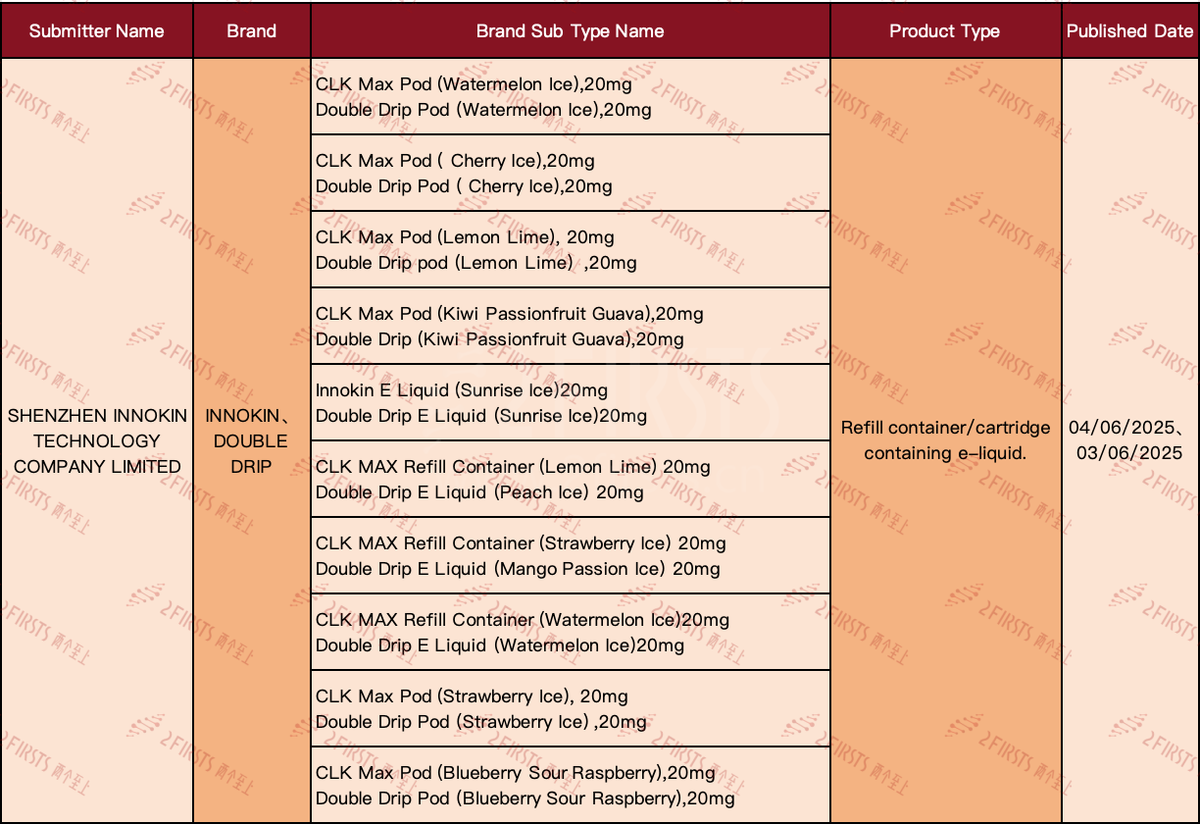
·Innokin and Double Drip brands simultaneously announced, covering a wider market or product niche.
·The menu offers a wide variety of flavors, including various fruits and refreshing tastes.
·All advertised products clearly label the nicotine content as 20mg/ml.
FUMOT and RELX Update Interchangeable Pod SKUs; VAPORLAX Unveils New Open-System Lineup
The e-cigarette brands FUMOT and RELX showcased their products, FUMOT MATE POD (DEVICE) and INFINITY 2, on June 4th and June 3rd, 2025 respectively. Both of these products are refillable and rechargeable e-cigarettes with interchangeable pods.
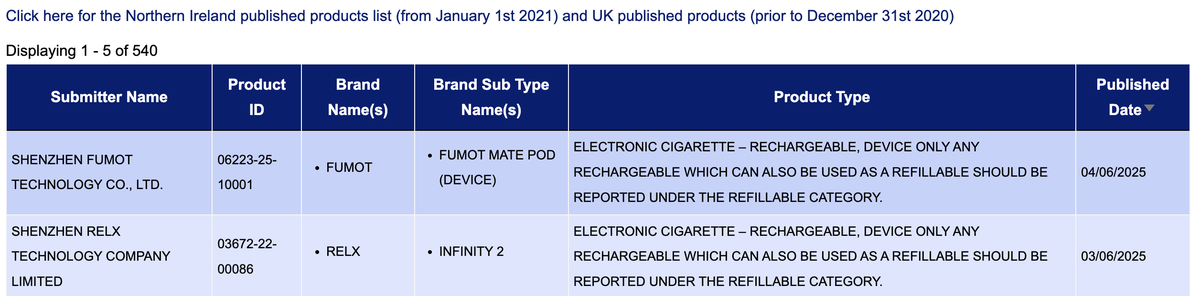
The e-cigarette brand VAPORLAX announced this week the release of two open system kits, the Vaporlax Nano Kit and Vaporlax Kubic Kit. These products do not come pre-filled with any substances and are considered refillable e-cigarette devices. In the category of "Individual part of electronic cigarette capable of containing e-liquid," the brand also announced the Vaporlax Nano Pod and Vaporlax Kubic Pod, which can be used in conjunction with the aforementioned devices.
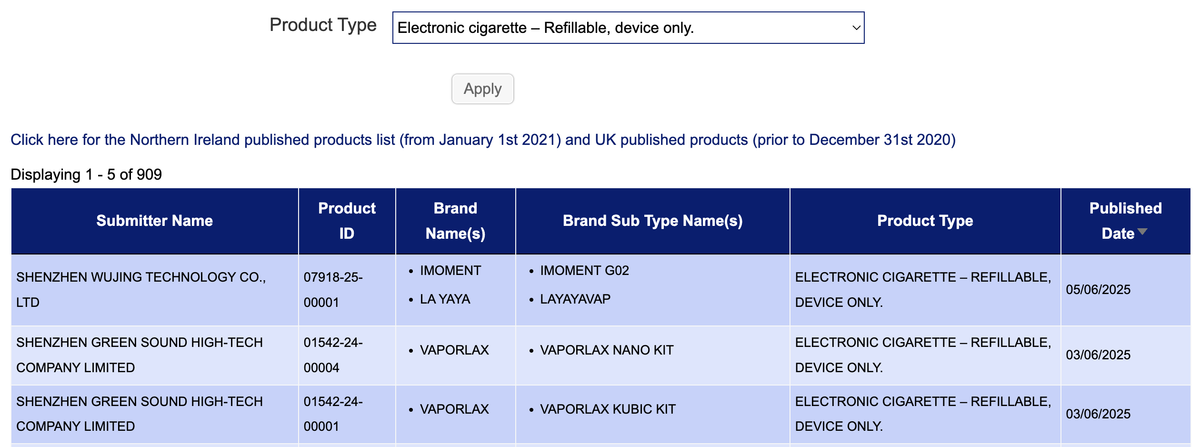
In addition, two open system devices, iMoment G02 from the IMOMENT brand and LAYAYAVAP from the same company's LAYAYA brand, were also showcased.
As a leading global NGP media and think tank, 2Firsts is committed to providing the latest product and technology information and insights for professionals in various categories such as e-cigarettes, heated tobacco, and modern oral products. We strive to promote technological changes and innovations in NGP products globally, ultimately bringing safer products and lifestyles to tobacco consumers worldwide.
With its information sources covering the Chinese supply chain and global markets, 2Firsts' product reporting has become one of the most influential global platforms for new product and technology releases.
Contact 2Firsts for:
1. Providing leads on new products and technologies;
2. Offering comments on products and technologies;
3. Seeking media coverage for your products;
4. Gathering sales channels for your products;
...
Contact information:
1.Email: info@2firsts.com
2.Connect with 2Firsts CEO Alan Zhao on LinkedIn.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







