
Disclaimer:
This article was contributed by the author to 2Firsts. The views and opinions expressed herein are solely those of the author and do not represent the position of 2Firsts. 2Firsts publishes this article to promote information exchange and diverse discussion. Readers are advised to exercise independent judgment when interpreting or citing its content.
Key Points:
- Dr. Nveed Chaudhary, Chair of GINN’s Scientific Committee, warns in a 2Firsts op-ed that a proposed COP11 ban on nicotine pouches would undermine global tobacco harm reduction efforts.
- Citing successful case studies from Sweden and Saudi Arabia, the article argues that properly regulated nicotine pouches can support smoking cessation and serve as a legitimate public health tool.
- The author critiques a leaked EU COP11 draft that pushes for restrictions without distinguishing between combustible and non-combustible nicotine products, potentially setting a problematic global precedent.
- He calls for globally aligned product standards and strong enforcement to prevent irresponsible high-dose pouch marketing that could damage the category’s credibility.
- Chaudhary concludes that rejecting harm reduction outright would entrench cigarette smoking, urging policymakers to adopt risk-proportionate regulation instead of blanket bans.
This article contains a wealth of research findings and is highly recommended for saving and re-reading. Key highlights include:
- Regulatory Classification of Nicotine Pouches Across 57 Countries: Drawing on updated data from Duren et al., the article categorizes countries into four regulatory approaches—regulated markets, unregulated grey markets, pharmaceutical-only pathways, and fully unregulated—providing a global policy snapshot at a glance.
- Relative Cancer Risk Assessment of Nicotine Pouches: Referencing a 2022 meta-analysis by Murkett et al., the article notes that nicotine pouches carry only 0.1% of the lifetime cancer risk of cigarettes (Relative Risk = 0.1), directly challenging the “no safe level” rhetoric.
- Case Studies from Sweden, Saudi Arabia, and the UK: Data from Saudi Arabia show a 41% full quit rate and 38.1% partial quit rate under strict state-controlled regulation. The UK’s public health authorities and the Royal College of Physicians endorse a “risk-proportionate” approach—signaling growing international consensus on tobacco harm reduction.
Nicotine Pouches at the Crossroads:
Why Prohibition at COP11 Would Be a Global Public Health Mistake
Dr Nveed Chaudhary
Chair, Scientific & Standards Committee, Global Institute for Novel Nicotine (GINN)

1、The Stakes at COP11
The Conference of the Parties (COP) is the governing body of the World Health Organisation’s Framework Convention on Tobacco Control (WHO FCTC), bringing together nearly 200 countries to shape global tobacco control policies[1]. The upcoming meeting in Geneva, Switzerland, represents a pivotal moment in the evolution of nicotine regulation. Binding decisions made here will not only influence how governments tax, market, and restrict tobacco and nicotine products but also determine the extent to which tobacco harm reduction is recognised as a legitimate public health strategy.
For millions of smokers this meeting could determine whether less harmful, smoke-free alternatives like nicotine pouches continue to be recognised as part of the quitting solution or restricted, which will increase the severity of the quitting challenge. What’s decided at COP11 will ripple across the world; it could drive product expansion and innovation that saves lives or set back progress by closing the door on established harm reduction options. The stakes are high because the choices made in Geneva will directly affect how quickly the world’s 1.3 Billion smokers[2] can move beyond smoking. The decisions made at COP have the potential to shape people’s health for generations, and the world will be watching.
2、Lessons from Success
Sweden’s experience offers a powerful example of how smoke-free alternatives can transform public health[3]. Through the widespread use of snus and, more recently, tobacco-free nicotine pouches, Sweden has achieved what no other nation has – smoke-free status, defined by the WHO as adult smoking rates under 5%. This success didn’t come from prohibition, but from providing smokers with realistic, lower-risk options[4].
A similar pattern is emerging elsewhere. A 2025 narrative review from King Abdulaziz University highlights the rapid growth of high-quality nicotine pouches in Saudi Arabia (KSA), where local company Badael are producing non-combustible products that meet adult demand whilst upholding public health standards[5]. The review concludes that nicotine pouches, when responsibly manufactured and marketed, offer a credible harm reduction pathway and should be seen as part of a balanced tobacco-control strategy and not a threat to it.
3、The EU Commission’s Leaked Proposal
Despite the positive net public health benefits demonstrated in Sweden and KSA, a leaked draft position attributed to the European Commission ahead of the WHO FCTC COP11 suggests that the EU may push for “strict regulation or bans” on smoke-free products, including nicotine pouches[6].

The EU draft position for COP11 goes beyond tightening product standards. It moves to explicitly challenge “harm reduction” and “reduced risk” marketing claims, treating such evidence-based communication as potential regulatory loopholes that need to be closed[7]. This shift reflects a worrying drift away from risk-proportionate public-health policy, blurring the regulatory distinction between combustible and non-combustible nicotine products.
Whilst unconfirmed, multiple reports indicate this stance could shape the Union’s common negotiating line in Geneva[8, 9]. Supporters argue a ban would protect youth, close regulatory gaps, and uphold the precautionary principle (taking preventive action in the face of scientific uncertainty). Critics warn it will likely undermine tobacco harm-reduction gains, especially where smoke-free products have been credited with creating steep declines in smoking, and risk shifting consumers back to cigarettes. Given that several member states are already exploring or enacting national prohibitions (e.g. France recently banned nicotine pouches[10] and imposed a penalty which is more severe than the illicit trading of heroin), COP11 could set a de facto template with a global ripple effect for policy, industry and most importantly, smokers’ health.
It is not hard to imagine the EU Commission’s logic in pushing for this nicotine pouch ban. The media narrative is steadily amplifying alarming accounts of youth access and misuse: recently, in the UK, reports surfaced of children “getting their hands,” on nicotine pouches [11]. In Montreal, Canadian researchers warned of rising teenage uptake[12]. Recently in the UK, watchdogs and retailers have sounded the alarm over “rogue” grey-market pouch sales, unregulated products bypassing merchant safeguards[13]. Meanwhile, toxicology journals have published studies on poisoning risks to young children who unintentionally ingest pouches[14].
From the Commission’s perspective, this is a confluence of regulatory failure (poor oversight, online sales, shadow brands) and public outcry (parents, media, child safety advocates). A prohibition or strong ban becomes politically attractive; it signals zero tolerance for youth risk, fills a perceived enforcement gap, and offers a broad stroke solution. More deeply, it aligns with the Commission’s precautionary instincts and with pressures to treat all nicotine delivery as inherently dangerous, especially when “unlicensed,’ noncombustible forms threaten to complicate the traditional tobacco control paradigm.”
If COP11 endorses a “ban or severe restriction” message, the EU’s narrative, built on youth protection, consumer risk, and regulatory chaos, may spread globally, as other regions view the EU as the regulatory subject matter experts on this issue
If adopted, the EU draft’s rejection of reduced-risk claims would remove a lawful, evidence-based tool from public-health frameworks and slow progress towards reducing smoking-related disease. Blanket suppression of accurate comparative information would weaken consumer choice, restrict regulated market pathways, and ultimately frustrate efforts to encourage smokers to switch to lower-risk alternatives.
4、WHO’s Failure to Uphold Its Own Framework
The WHO’s interpretation of its own treaty is inconsistent with the FCTC it claims to defend. Article 1(d) of the FCTC explicitly defines tobacco control as including harm-reduction strategies. Yet, WHO’s guidance for COP11 continues to marginalise these approaches.
This contradiction appears to undermine public-health outcomes: a prohibitionist stance removes the opportunity for smokers to switch to less harmful products and leaves countries without workable harm-reduction strategies at a disadvantage. In Sweden and the UK, pragmatic integration of safer nicotine products such as pouches and e-cigarettes have been pivotal in driving smoking rates to historic lows. Ignoring such evidence risks further entrenching or reviving the very combustible tobacco use the FCTC was designed to eliminate.
5、Expert Evidence Refuting WHO’s Prohibitionist Logic
Independent public-health experts have repeatedly challenged WHO’s prohibitionist framing of nicotine. Clive Bates (The Counterfactual) has cautioned that the WHO’s hostility towards innovative, non-combustible nicotine formats risks pushing consumers back to cigarettes[15]. He has emphasised that nicotine itself is not the cause of tobacco-related disease and that more than 60% of smokers still mistakenly believe nicotine causes cancer. Such misperceptions lead many to assume alternative nicotine products are just as harmful as cigarettes, thereby discouraging switching to less harmful options. The Global State of Tobacco Harm Reduction (GSTHR) has documented how less harmful nicotine products, such as vapes or pouches, separate nicotine from the toxins of combustion, offering a life-saving opportunity for hundreds of millions of smokers[16]. GSTHR warns that WHO’s continued resistance to harm reduction “fights the last war” and undermines global health progress. The UK’s Office for Health Improvement and Disparities (OHID) affirms that while not risk-free, vaping and oral nicotine products pose only a small fraction of the risks of smoking[17]. OHID and the Royal College of Physicians both urge health professionals to support complete switching to less harmful alternatives rather than perpetuating nicotine misinformation[18]. The U.S. FDA’s 2025 authorisation of 20 Zyn nicotine pouch products confirms the risk-proportionate approach, recognising them as being appropriate for the protection of public health (APPH)[19].
Collectively, this body of evidence demonstrates that WHO’s “no safe level of nicotine” rhetoric is scientifically unsound. Nicotine is addictive, but is not the cause of smoking-related disease. Effective global policy must distinguish between combustion and non-combustion and regulate products according to risk not ideology.
6、The Real Problem
In many parts of the world, we are seeing a surge of nicotine pouch brands entering the market. While the large multinational manufacturers generally, and the up-and-coming serious nicotine pouch only manufacturers tend to operate with strong compliance and a commitment to best practice, several smaller, short-lived brands have adopted marketing behaviours that conflict with public health principles and fail to treat nicotine with the respect due to an addictive substance.
There is no question that nicotine pouches and all nicotine products should not be used by nicotine-naïve youth or adults who have otherwise never used a nicotine-containing product. Their purpose should be clear: to provide adult smokers with a far less hazardous alternative to combustible tobacco. Using nicotine pouches eliminates burning, eliminates tobacco and eliminates inhalation, leaving a delivery mechanism comparable to pharmaceutical nicotine pouches, gum or lozenges, which are approved and regulated medicinal products in many countries.
The WHO recognises nicotine replacement therapy (NRT) as part of its Model List of Essential Medicines for the indication of Nicotine dependence[20]; a designation reserved for treatments considered fundamental to meeting the most important health needs of populations. The irony is striking: it is this same organisation, through COP11, that now stands to influence policies that could restrict access to one of the most effective harm-reduction tools ever developed.
Furthermore, nicotine pouches have existed in the Nordic regions for over a decade as recognised NRTs, demonstrating that, when supported by appropriate regulation and responsible oversight, the format can coexist safely within a public-health framework. The issue, therefore, lies not with the product itself but with the aggressive commercial strategies and absence of proportionate regulation to counterbalance them.
This moment demands a treaty-based critique grounded in evidence and legal consistency. Any expansion of marketing or product restrictions must be justified by data within a societal context, not data viewed in a biased silo or through political optics. Independent toxicology and epidemiology consistently show vast relative-risk differences between combustible and non-combustible nicotine products. Public-health policy should therefore create product-specific regulatory pathways, not impose horizontal bans that erase distinctions between deadly and less harmful options.
7、Setting Sensible Limits
In an ideal world, every company operating in this space would act responsibly. But recent examples show that this is far from reality. The excitement surrounding exponential market growth and the race for rapid sales has led a few short-lived brands to lose sight of what truly matters: tobacco harm reduction and the opportunity to save millions of smokers’ lives.
A handful of companies have released products containing 100-150mg of nicotine per pouch; a reckless and unjustifiable marketing tactic clearly designed to attract attention and drive sales through dangerous differentiation, rather than help smokers quit. Most anecdotal evidence indicates that most adult smokers achieve satisfaction and craving relief with pouches containing 9-15mg of nicotine. Even the heaviest smokers are very unlikely to require more than 20mg per pouch. A study by Chapman et al. confirmed that there was no meaningful difference in satisfaction, craving relief, or psychological reward between pouches containing 14mg, 16mg, or 20mg of nicotine. Even amongst experienced snus users, pouches at 16-20mg delivered the same satisfaction as traditional snus at 16.6mg[21].
There is no scientific justification for nicotine pouches exceeding 20mg per pouch. Such products do nothing to support adult smokers in quitting; they only invite regulatory backlash and undermine the credibility of the entire category. High nicotine doses are toxic; there is no legitimate reason to include them when lower strengths are equally effective.
8、The Need for Global Product Standards
The problem, therefore, lies not with the product itself, which can serve as a legitimate and effective vehicle for smoking cessation, but with the way in which it is marketed and the absence of robust product standards at regional, continental and global levels. Without clear regulatory frameworks, enforcement becomes almost impossible, largely because no single authority has the jurisdiction or mandate to act.
A compelling example comes from the U.S. Food and Drug Administration (FDA), which operates one of the world’s most stringent regulatory pathways for non-pharmaceutical nicotine products: the Premarket Tobacco Product Application (PMTA). The FDA has explicitly acknowledged the public-health benefits of nicotine pouches, recognizing their potential to reduce smoking-related harm even beyond that of heated tobacco, vapes or snus[22]. In response, the agency recently introduced a streamlined, fast-track review process for nicotine pouch applications, an important step toward ensuring that only authorised, evidence-based products remain on or enter the market.
However, such authorisation systems only retain their credibility if enforcement is firm and reliable. Allowing unauthorised products to proliferate undermines both consumer trust and the integrity of harm-reduction policy. Regulatory approval must therefore be matched with decisive action against non-compliant products and retailers (including online), to deter abuse and protect the long-term credibility of this emerging category.
The Kingdom of Saudi Arabia (KSA) is a case in point. Although the only available nicotine pouch is produced by a private company under the government’s Public Investment Fund (PIF), the regulatory framework is firmly state-controlled. Current regulations prohibit the import or sale of other brands, resulting in a tightly governed marketplace with clear rules on manufacturing, marketing and distribution.
The results have been remarkable. In less than two years, among pouch users, 41.0% reported quitting smoking and 38.1% reported partial quitting[23]; furthermore, another study of adults in Riyadh found that 95.8% of oral nicotine pouch users were current or former users of cigarettes or other inhalable nicotine products[24]. This experience shows that when regulation is clear, enforcement is strong, and rogue players are excluded, nicotine pouches can deliver a profound public-health benefit. A controlled, standards-based market doesn’t suppress innovation; it channels it toward the collective goal of a healthier, smoke-free society.
Two further examples within the EU illustrate sensible, pragmatic, and evidence-based approaches to nicotine pouch regulation. Both Greece and the Czech Republic have recently introduced clear standards and nicotine caps designed to balance public-health protection with harm-reduction potential.
In Greece, a May 2025 proposal introduced a 16mg nicotine cap per pouch and strengthened the ban on sales to minors, with criminal penalties for offending retailers. Flavour options have been restricted to tobacco, menthol and mint, and a special consumption tax of €50 per kilogram, plus 24% VAT, has been applied.
The Czech Republic, by contrast, implemented a comprehensive regulatory framework in 2023, establishing a 12mg nicotine cap, a defined list of prohibited additives, detailed labelling and packaging requirements (including a ban on resemblance to food, cosmetics or toys), and mandatory child-resistant packaging. Sales are restricted to licensed retailers only, ensuring product integrity and accountability across the supply chain.
An alternative regulatory pathway adopted in some countries is pharmaceutical licensing. Nations such as India, Canada, and Australia have classified nicotine pouches as medicinal nicotine replacement therapies (NRTs), with strict dosage limits and distribution controls. India, for example, caps nicotine content at 4mg per pouch for prescription pouches. Yet this concentration is too low to help many heavy smokers quit, rendering these products largely ineffective as true harm-reduction tools.
While these regulatory changes are still too recent to assess their full impact on smoking prevalence in either country, early market indicators suggest rapid uptake of pouches. In 2023, a snapshot forecast projected 15% year-on-year growth in the nicotine pouch market, reflecting strong consumer transition toward lower-risk nicotine alternatives.
While pharmaceutical designation creates a highly controlled marketplace, it also stifles innovation in product design, flavor development and materials used. It does more to protect nicotine naïve users than to support adult smokers seeking less risk alternatives. Moreover, because smokers often do not identify as patients, pharmacy purchases greatly reduce convenience, uptake, and ultimately switching from cigarettes.
Many countries around the world do not have clear regulations for nicotine pouches, and some have simply banned them without consideration of the harm reduction benefits they could bring. Table 1, adapted and updated from Duren et al. shows four different approaches to the policies regulating nicotine pouches in a sample of 57 countries around the world[25].
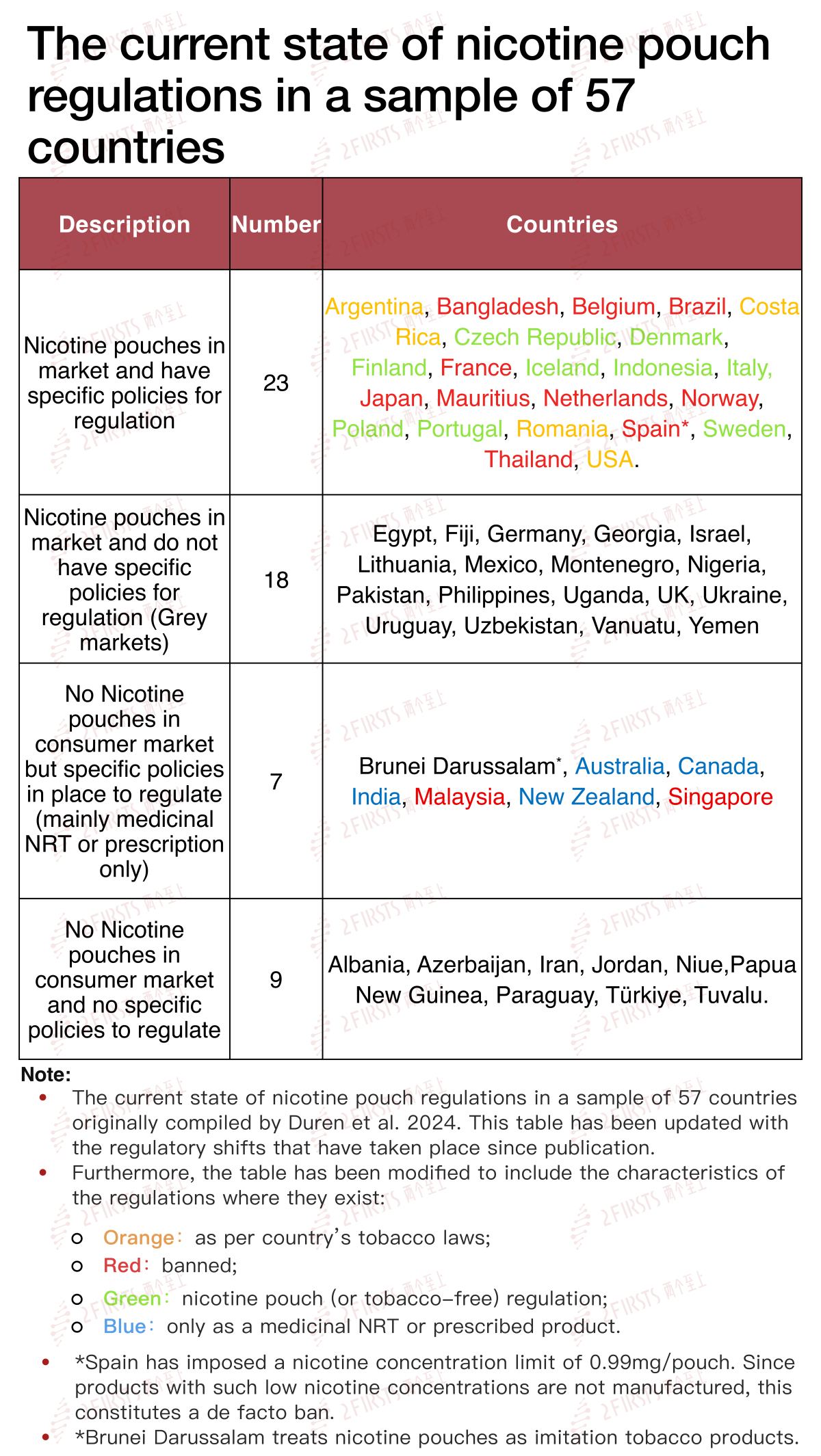
9、An Experienced Warning to the EU
Sweden, where nicotine pouches first emerged as a modern evolution of traditional snus, has already witnessed their powerful contribution to achieving a smoke-free society. Drawing on this success, Sweden has urged fellow EU member states, including France, Belgium, the Netherlands, Germany and Spain, where outright bans are already in place, to reconsider their approach. The Swedish government has warned that imposing further national or EU-wide prohibitions would be a grave mistake, as such actions “completely ignore the harm reduction perspective” that underpins modern public health policy[26].
The UK’s position remains unclear. Although no longer part of the EU, the UK’s Tobacco and Related Products Regulation (TRPR) mirrors the EU Tobacco Products Directive (TPD), and neither framework currently regulates tobacco-free, non-pharmaceutical nicotine pouches. Legally, it is still not prohibited for minors to purchase them. This regulatory vacuum makes the EU Commission’s new call for prohibition appear contradictory. Having ignored the need for proportionate regulation, it now seeks to ban the very products that could save smokers’ lives by offering a safe, high-quality and legal alternative.
It appears that the perfect storm is forming in the UK, one that, if navigated shrewdly, could set a global standard for nicotine-pouch regulation. The Tobacco and Vapes Bill is currently under parliamentary consideration, seeking powers to regulate tobacco, vaping, and “other products.” In parallel, July 2025, saw the Department of Health and Social Care (DHSC) launch its 10-year health plan[27], whose flagship ambition is to “deliver on our world-leading Tobacco and Vapes Bill” under the banner “from sickness to prevention.”
If the DHSC insists on proportionate, science-based regulation of nicotine pouches embedded in the Bill, the UK could establish a safe, controlled marketplace and still pursue its smoke-free objectives. The opportunity is to give UK smokers access to the lowest-risk, non-pharamceutical, and acceptable nicotine alternative, aiding transition away from combustibles. But if policymakers fail to integrate these provisions, or overly restrict them, the UK risks repeating the regulatory voids that are current, and with a prohibitionist stance from the EU, leaving smokers with no viable path forward.
10、The Hypocrisy of Banning the Least Harmful Product
If the European Commission and, by extension, the WHO wish to advocate for bans, then let them begin by banning combustible cigarettes.
A recent meta-analysis published by Murkett et al (2022), compared the relative lifetime cancer risk of 15 nicotine products, using emission and content analyses for twelve Group 1 carcinogens[28]. Taking combustible cigarettes as the benchmark at 100, the study found that nicotine pouches carry a relative risk of just 0.1, a reduction of approximately 1,000-fold.
It is therefore illogical and counterproductive that, having long neglected to regulate nicotine pouches proportionately, governments now move to suggest prohibiting the least risky form of nicotine consumption while continuing to sanction the most lethal. Such inconsistency not only undermines public-health credibility but also betrays the scientific evidence on which rational regulation should be based.
The usual argument against banning cigarettes is that it would fuel the illicit market. Yet to suggest that banning nicotine pouches would not create the same dynamic is disingenuous. Consumers will always seek alternatives, and prohibition only pushes demand underground, where quality, safety, and age control vanish entirely.
Perhaps the uncomfortable truth lies elsewhere. The vast tax revenues that governments derive from cigarette sales. To ban combustibles would mean forfeiting billions in annual income. But if the true goal of the WHO FCTC is to protect public health, then logic dictates a different path: phase out combustible tobacco while allowing a strictly regulated, science-based marketplace for safer nicotine alternatives. Anything less exposes a double standard that undermines both the credibility and moral authority of global tobacco control.
11、Conclusion
Especially in countries identified in Table 1 as “grey markets,” and those where nicotine pouches have yet to gain meaningful traction and where regulatory guidance remains absent, there lies a significant opportunity to replicate the success already demonstrated in Sweden and KSA. By positioning nicotine pouches as the least harmful and most accessible form of nicotine delivery, millions of smokers could be supported in transitioning away from combustible products.
However, this potential can only be realized through the establishment of a comprehensive and globally aligned framework, giving manufacturers a clear and consistent target. The principle of Aligned Innovation ensures that such a framework is science-led, regulator-aligned, and consumer-focused. A framework calling for a nicotine ceiling of 20mg/pouch, child-resistant packaging, responsible labelling, regulated adult-only marketing and preferential tax treatment of all nicotine products commensurate with harm seems like logical elements for such a framework.
Crucially, regulation must be matched by robust enforcement. Without credible oversight and meaningful manufacturer, consumer and retailer (including online) penalties for non-compliance, regulation is little more than guidance on paper. Effective enforcement protects consumers, rewards responsible manufacturers, and ensures that innovation serves public-health goals rather than undermining them.
This balanced approach gives smokers the best possible chance of success in their quit journeys while providing the industry with clear, enforceable guardrails within which to operate legitimately while generating a fair profit. Only once such a system exists, and if, despite it, certain actors persist in acting irresponsibly and uncontrollably, should the notion of prohibition even be entertained.
Rejecting harm reduction outright would not advance public health; it would further entrench smoking and perpetuate preventable disease, putting at risk the lives of the millions who might benefit.
About the Author
Dr. Nveed Chaudhary is a scientist and strategist with over 20 years of experience in the alternative nicotine products space, specialising in innovation, regulatory science, and harm reduction. He is the Founder and Managing Director of NexCentra Consulting, advising global companies on next-generation nicotine and pharmaceutical products. His work bridges science, regulation, and public health to enable responsible, evidence-based innovation.
The above biography and materials were provided by the author.
References
1. WHO. COP11 - 20 Years of Change. Uniting Generations for a Tobacco-Free Future. 2025 15.10.2025]; Available from: https://fctc.who.int/convention/conference-of-the-parties/sessions/eleventh-session-of-the-conference-of-the-parties.
2. WHO. Tobacco: Key Facts. 2025 25.06.2025 [cited 2025 22.10.2025]; Available from: https://www.who.int/news-room/fact-sheets/detail/tobacco?
3. Sweden), F.P.H.A.o. Use of Tobacco and Nicotine Products. 2024 12.12.2024 15.10.2025]; Available from: https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/living-conditions-and-lifestyle/andtg/tobacco/use-of-tobacco-and-nicotine-products/.
4. Foulds, J., et al., Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tob Control, 2003. 12(4): p. 349-59.
5. Al-Otaibi, H.M. and M.A. Althobiani, Nicotine pouches: a narrative review of the existing literature. Front Public Health, 2025. 13: p. 1641308.
6. Anderson, A. EU’s Leaked COP11 Position SIgnals Harsh Crackdown on Safer Nicotine Products. 2025 8.10.2025 [cited 2025 15.10.2025]; Available from: https://clearingtheair.eu/en/post/exclusive-eus-leaked-cop11-position-signals-harsh-crackdown-on-safer-nicotine-products/.
7. Council, G.S.o.t., 11th Conference of the Parties (COP11) to the WHO Framework Convention on Tobacco Control (WHO). 2025, Council of the European Union: Brussels.
8. Alliance, W.V. COP11: EU Commission Opens Door for Full Vape and Nicotine Poiuch Ban in Europe. 2025 09.10.2025 [cited 2025 15.10.2025]; Available from: https://finance.yahoo.com/news/cop11-eu-commission-opens-door-142600974.html?guccounter=1&guce_referrer=aHR0cHM6Ly93d3cuZ29vZ2xlLmNvbS8&guce_referrer_sig=AQAAADTpgppchK_4XnqzMMIaeeMzkHQblZlM1lUgYrGqNtQyGNstGmKzKIKcuBxqHM7oHR9QHjzkzjKDY2N5mVVIValuj5uPuSKaX9ML2OWy9ZnUw1LxwNLe0Bl76NzmXqpy7IwS1tBtgIS-74uTNcNqs1SOjHW5H97Dx9qFqHzqd-fc.
9. 2Firsts. Leaked EU Paper Suggests COP11 Puch to Ban Nicotine Pouches and Flavoured Nicotine Products. 2025 10.10.2025 [cited 2025 15.10.2025]; Available from: https://www.2firsts.com/news/leaked-eu-paper-suggests-cop11-push-to-ban-nicotine-pouches-and-flavoured-nicotine-products.
10. Casassus, B., Nicotine pouches banned in France. BMJ, 2025. 390: p. r1895.
11. Holt, J. Children are getting their hands on nicotine pouches and fainting. 2025 19 Sep 2025 [cited 2025 19 Sep 2025]; Available from: https://www.manchestereveningnews.co.uk/news/greater-manchester-news/children-getting-hands-nicotine-pouches-32507984.
12. Battista, K., et al., Emerging use of oral nicotine pouches among Canadian adolescents: Findings from the COMPASS-Quebec study. Can J Public Health, 2025.
13. Kennedy, J. Scottish Grocers’ Federation issues retailer advice on nicotine pouches. 2025 [cited 2025 15.10.2025]; Available from: https://www.conveniencestore.co.uk/news/scottish-grocers-federation-issues-retailer-advice-on-nicotine-pouches/710395.article.
14. Anderer, S., Nicotine Pouch Ingestions Surge Among Young Children. JAMA, 2025. 334(9): p. 758.
15. Bates, C., Prohibitionists at work: how the WHO damages public health through hostility to tobacco harm reduction, in The Counterfactual. 2021: London.
16. Knowledge.Action.Change, Fighting the Last War: The WHO and International Tobacco Control, ed. R. Goldstein. 2021, London.
17. Disparities, O.f.H.I.a., Nicotine vaping in England: an evidence update including health risks and perceptions. 2022: London.
18. Physicians, R.C.o., E-CIgarettes and Harm Reduction: An Evidence Review. 2024, London.
19. FDA, FDA Authorizes Marketing of 20 ZYN Nicotine Pouch Products after Extensive Scientific Review. 2025, FDA: Washington.
20. Organisation, W.H. Model List of Essential Medicines. 2025 [cited 2025 15.10.2025]; Available from: https://list.essentialmeds.org/.
21. Chapman, F., et al., Evaluation of high-nicotine oral products shows potential to reduce tobacco-related harm by offering satisfying alternatives. Sci Rep, 2025. 15(1): p. 34636.
22. Products, C.f.T. FDA Launches Program to More Efficiently Review Nicotine Pouch Applications. 2025 18.09.2025 [cited 2025 15.10.2025]; Available from: https://www.fda.gov/tobacco-products/ctp-newsroom/fda-launches-program-more-efficiently-review-nicotine-pouch-applications.
23. Alwafi, H., et al., Prevalence, safety, and role of nicotine pouches in smoking cessation among smokers and the public in Saudi Arabia. Sci Rep, 2025. 15(1): p. 29758.
24. Alkharaan, H., et al., Investigating oral nicotine pouch use among adults in Riyadh, Saudi Arabia: prevalence, awareness, susceptibility, and associated symptoms. Front Public Health, 2025. 13: p. 1607656.
25. Duren, M., et al., Nicotine pouches: a summary of regulatory approaches across 67 countries. Tob Control, 2023.
26. Michalpoulos, S. Sweden moves to stop nicotine pouches meeting the fate of snus. 2025 09.10.2025 [cited 2025 15.10.2025]; Available from: https://www.euractiv.com/news/sweden-moves-to-stop-nicotine-pouches-meeting-the-fate-of-snus/.
27. Care, D.o.H.a.S., FIt For The Future, D.o.H.a.S. Care, Editor. 2025, UK Government: London.
28. Murkett, R.R.M., Ding, B, Nicotine products relative risk assessment: an updated systematic review and meta-analysis [version 2; peer review: 1 approved, 1 approved with reservations]. F1000Research, 2022. 9: p. 1225.
Reference
- Opinion | Samrat Chowdhery: Tobacco Price Elasticity—A Convenient Myth?

- Exclusive 2Firsts Contribution | Dr. Ziauddin Islam: Smoke-Free Pakistan — Dream or Reality?

- On the Global Tobacco Battlefield: The Clash Between Science and Misinformation — A Tobacco Harm Reduction Expert’s Contribution to 2Firsts










