The director of the U.S. Food and Drug Administration's Center for Tobacco Products, Brian A. King, shed light on a progressive three-pronged approach at the Global Tobacco & Nicotine Forum (GTNF) 2023, to nurture healthier consumer choices and instigate industry innovation concerning Reduced-Risk Products (RRPs) and traditional tobacco products.

Promoting Safer Alternatives and Age Restrictions
King underscored the importance of steering consumers away from riskier habits towards safer RRPs through behavioral support programs.
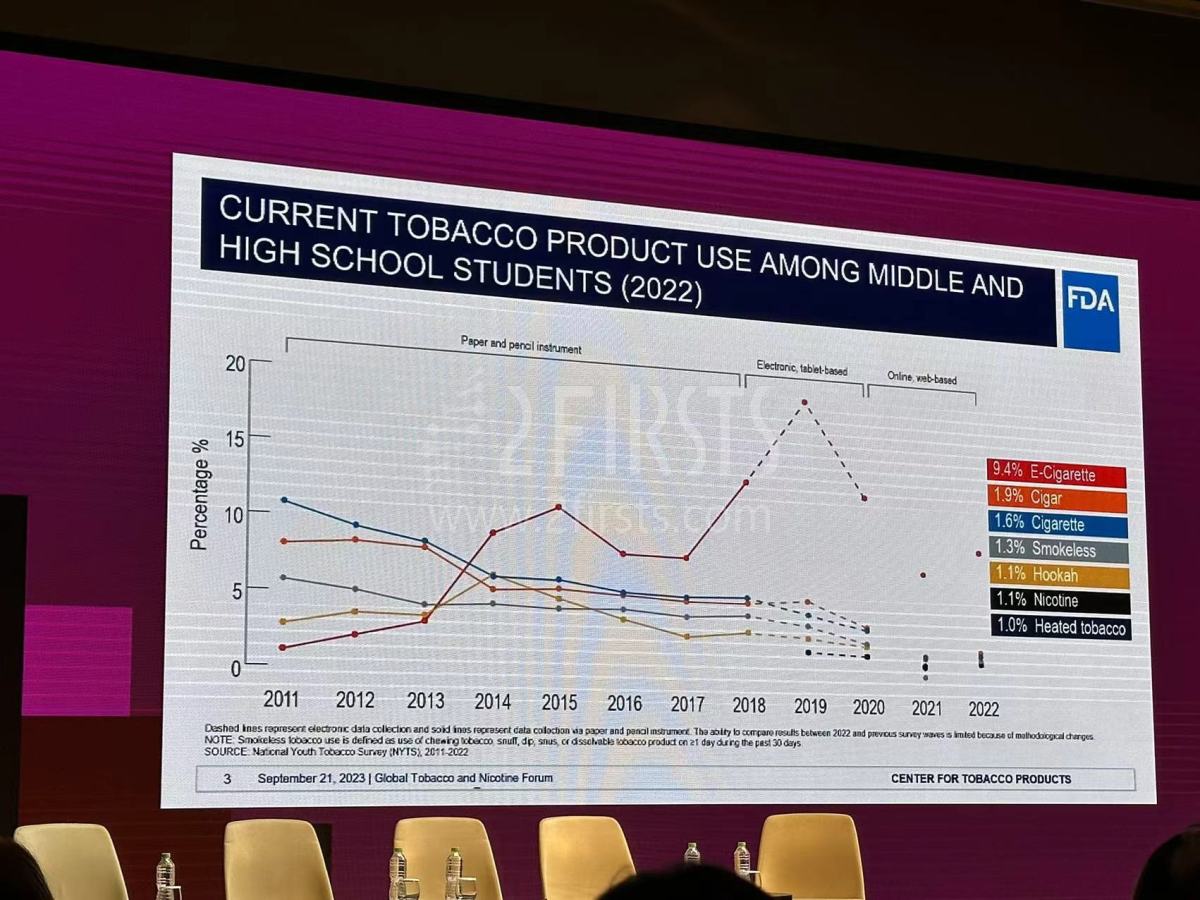
The economic toll of smoking is hefty, costing the U.S. over $240 billion in healthcare spending and $185 billion in lost productivity in 2018. Moreover, a 2022 survey revealed a worrying 9.4% e-cigarette usage rate among middle and high school students, topping the list of tobacco products used by this demographic. To combat youth initiation issues, he stressed the implementation of strict age restrictions on device and product usage.
To curb youth access to harmful products, the FDA issued warnings against flavored, disposable vapes, and carried out nationwide inspection blitzes of retailers and distributors. As of August 11, 2023, over 1200 warning letters were dispatched through online investigations for various tobacco product violations, underlining the FDA's ongoing commitment to enforcing compliance and promoting public education on the matter.
Differentiating RRPs from Conventional Tobacco Products
A clear-cut regulatory differentiation between RRPs and conventional tobacco products is essential, opined King. Citing Greece's varied regulatory stringency on labeling, advertising, and packaging as an example, King highlighted how such measures aid consumers in making informed decisions based on associated risks.
He also touched on the evolutionary phases of the tobacco and nicotine product industry in the U.S., ranging from the highly concentrated pre-2006 era to the recent developments post the rise of e-cigarettes around 2018, leading to a landscape ripe for innovative nicotine delivery products.
Constructive Policy Stance Including Regulatory and Fiscal Measures
The proposal also involved leveraging both regulatory and fiscal measures to spur industry innovation towards potentially less harmful products. King drew parallels with other industries like the fuel market and beverage industry, illustrating how product specification requirements could catalyze innovation and economic benefits while facilitating societal gains.
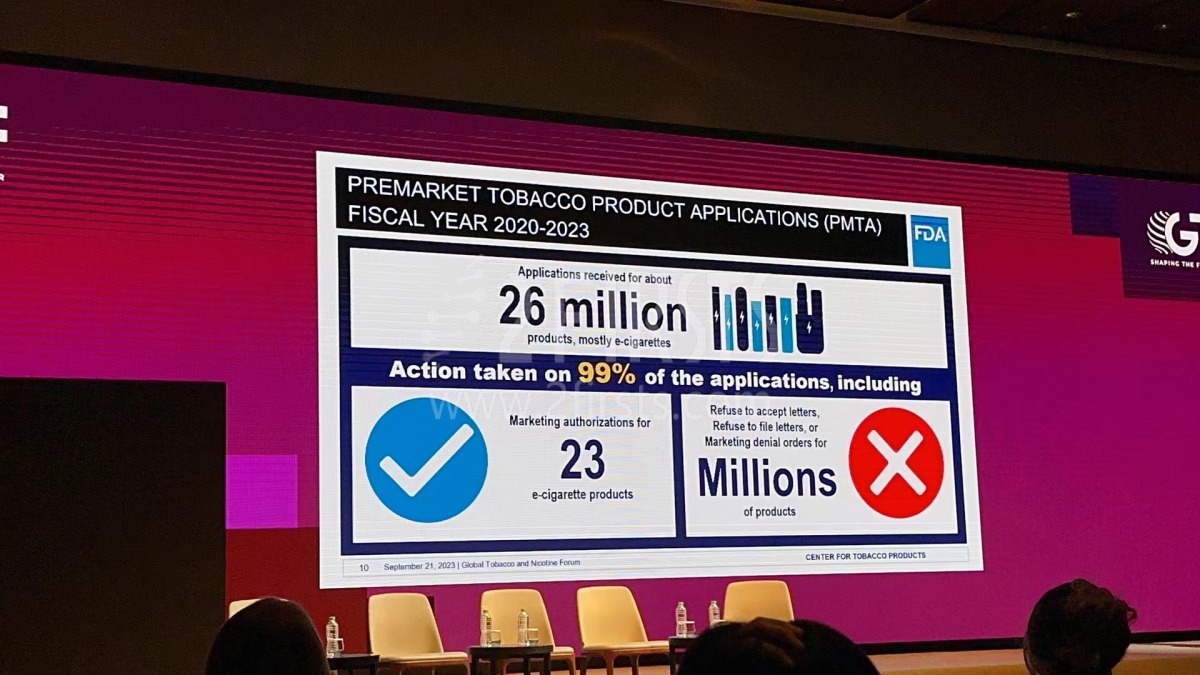
Since 2009, the FDA has been proactive in issuing tobacco control acts like the Tobacco Control Act (2009), Deeming Final Rule (2016), and Non-Tobacco Nicotine (2022). The Center for Tobacco Products, established to oversee tobacco product applications and enforce regulations, has seen a deluge of approximately 26 million PMTA applications between 2020 and 2023. A whopping 99% of these applications have been acted upon, authorizing 23 e-cigarette products while millions were refused acceptance.
With eternal evaluation actions encompassing a gamut of strategies from science and application review to compliance and public education campaigns, the FDA's outlined approach at GTNF 2023 signals a constructive pathway towards aligning consumer, industry, and societal interests in the face of the evolving global tobacco market.


