
On September 18th, British American Tobacco (BAT) fully-owned subsidiary RJ Reynolds Tobacco Company (hereinafter referred to as "Reynolds Tobacco") sent an email to distributors and wholesalers titled "FDA Enforcement Notice on Retail Sales of Elf Bar and Esco Bars e-cigarettes".
In a recent email, the US Food and Drug Administration (FDA) announced that on September 14th, they issued warning letters to 15 online retailers and 3 manufacturers and/or distributors for selling or distributing unauthorized e-cigarette products. These warning letters highlighted a range of popular e-cigarette products that appeal to young people, including disposable products from well-known brands such as Elf Bar, EB Design, Lava, Cali, Bang, and Kangertech.
Disposable Brands Called out
This is not the first time Reynolds Tobacco has sent out such emails. An anonymous e-cigarette distributor in the United States told 2FIRSTS that Reynolds Tobacco has previously sent several emails to distributors, alerting them about FDA announcements regarding product information.

Reynolds's email|Source: American distributor
This dealer stated that their collaborating retailers often receive "warning" emails from Reynolds Tobacco. Upon receiving these emails, the dealer or wholesaler would reduce the quantity of the brand's products involved during procurement. They may also stop selling these e-cigarette products to avoid FDA penalties.
Reynolds Tobacco listed multiple Chinese e-cigarette brands in a recent email, including ELFBAR, EBDesign, Kangertech, Lava, Cali, Bang, Esco Bars, Innokin, Puff Max, Hyde, and Puff Bar. The email also mentioned the popular disposable brand Breeze currently dominating the US market. Reynolds Tobacco cited a previous FDA announcement stating that these products are all disposable e-cigarette products that have not passed the Pre-Market Tobacco Application (PMTA) process.
Reynolds Tobacco concluded their email with the following statement, "Please contact your supplier or a representative from Reynolds to ensure you have an ample supply of Vuse Alto e-cigarette products, ensuring the satisfaction of adult nicotine consumers aged 21 and above."
It's worth mentioning that Vuse Alto is still under FDA review. This product was submitted nearly a year later than Vuse Solo and is currently in the application stage, yet to receive official approval.
Scheming of British-American Tobacco
In regards to this email, Zheng Zhi, PMTA expert and founder of Ziyuan Technology, stated to 2FIRSTS, "This presents a great opportunity for R.J. Reynolds Tobacco to promote its Vuse brand, as created by the FDA."
Zheng Zhi believes that Reynolds Tobacco intends to inform all retailers and wholesalers that the Vuse brand will not be subject to FDA warnings or bans. However, other brands, no matter how popular they may be, face a constant risk of being removed from shelves.
In 2021, Vuse Solo, a subsidiary of Reynolds Tobacco, became the first e-cigarette product to pass the PMTA and receive FDA certification. Vuse Solo has obtained e-cigarette marketing authorization and licensing.

British American Tobacco's celebratory announcement | Source: British American Tobacco
Mitch Zeller, the current director of the Center for Tobacco Products (CTP) at the FDA, openly stated, "According to the manufacturer's data, their tobacco-flavored products can benefit adult smokers who switch to these products by reducing their exposure to harmful chemicals - whether they completely quit smoking or significantly reduce their cigarette consumption."
The parent company of Reynolds Tobacco, British American Tobacco, sees this as a "significant regulatory achievement through PMTA." So far, a total of 9 products under the VUSE brand have successfully gone through PMTA, out of which only 23 e-cigarette products have met the requirements. Products from British American Tobacco's portfolio account for nearly 40% of the compliant quota.
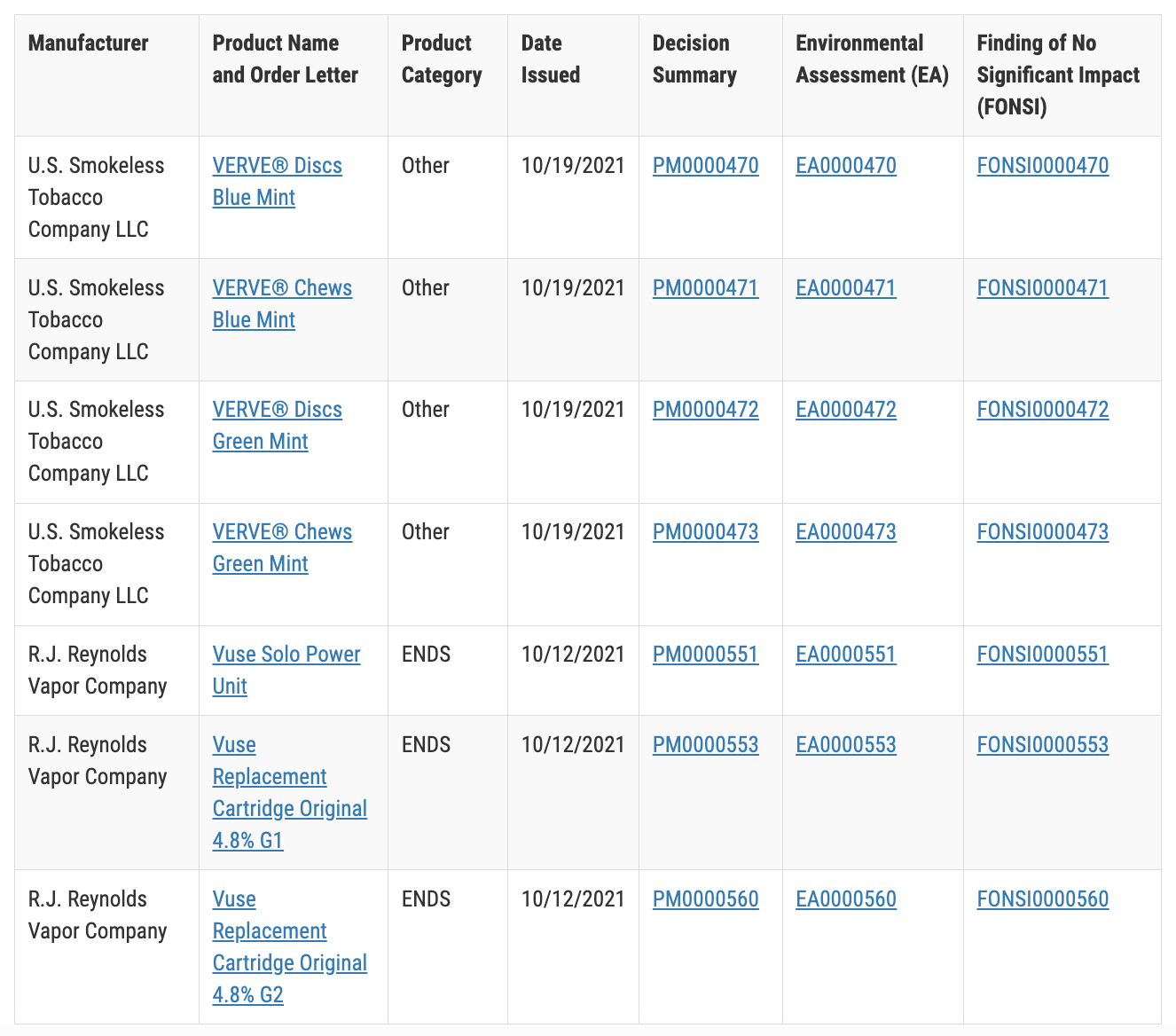
Vuse Solo is the first e-cigarette product to pass PMTA | Source: FDA
Zheng Zhi believes that such notifications (referring to emails) are something that Reynolds Tobacco really enjoys doing, as they frequently send them to their clients.
He considers compliance as an essential part of Raynolds Tobacco's marketing campaign.
On one hand, it is expressed that the customer service and after-sales service of Reynolds Tobacco are highly professional. On the other hand, they constantly remind customers to place more orders. After all, promoting "compliance" is also considered part of marketing.
In fact, 2023 has been a year of comprehensive strengthening of global e-cigarette regulations. The Center for Tobacco Products (CTP) has repeatedly emphasized the importance of "compliant products" in response to external pressures.
In August 2023, the Attorneys General of 33 states in the United States collectively wrote a letter to the FDA, demanding a ban on the sale of flavored e-cigarettes and intensified efforts to combat marketing targeted at adolescents. The following month, at a meeting of the National Association of Tobacco Outlets (NATO) in the US, Brian King, Director of the Center for Tobacco Products (CTP), stated that enforcement actions will continue to be directed towards retailers, with a focus on products that have not undergone Pre-Market Tobacco Product Application (PMTA) submission.

Signed letter | Source: cronkitenews
Moreover, this aligns with CTP's operational focus for the past two years, which is 'compliance, full compliance,'" added Zheng Zhi.
Full Implementation of PMTA Drawing Closer

DA issued marketing denial orders for approximately 6,500 flavored e-cigarette products | Source: FDA
Several "warning emails" from Reynolds Tobacco are based on FDA announcements. What is the main purpose behind FDA's multiple notifications this year?
According to Zheng Zhi's analysis, the FDA has never abandoned its original intention and has been constantly preparing for the comprehensive implementation of PMTA. Whether it's streamlining internal processes, personnel adjustments, initiating clinical trials, approving compliant products, establishing factory quality systems, or banning illegal products, all of these efforts and measures are aimed at the ultimate goal of making PMTA truly operational.
According to publicly available information, in May 2016, the FDA reviewed other tobacco products under the revised Family Smoking Prevention and Tobacco Control Act and the Federal Food, Drug, and Cosmetic Act. This rule grants the FDA the authority to regulate e-cigarettes and cigars.
However, the progress of PMTA review for e-cigarettes is slow, and the Reagan-Udall Foundation criticizes the CTP for operating primarily in a passive mode. As time goes on, the number of applications that need to be processed has increased significantly.
In March 2023, the FDA announced that it has made decisions on over 99% of the nearly 26 million submissions considered as products. The agency has issued Refuse to Accept (RTA) letters, refuse to submit letters, or rejected orders for millions of products.

Issued announcement | Source: FDA
Zheng Zhi believes that despite tremendous obstacles, the FDA is steadily making progress and its enforcement actions primarily target major brands.
The intention behind this is very clear, "On one hand, it aims to reduce law enforcement costs, and on the other hand, it serves as a deterrent for future offenses."
Zheng Zhi believes that with the increasing number and scale of actions taken by the FDA, it is foreseeable that the comprehensive and genuine implementation of PMTA is getting closer and closer.
In the foreseeable future, especially before PMTA has a conclusive outcome, retailers and wholesalers may continue to receive "warning" emails from Renuo Tobacco. After all, as the final link in the US e-cigarette industry, retailers directly determine which e-cigarette products consumers can see, and they are also direct participants in the submarket.
2FIRSTS has sent an email to Reynolds Tobacco regarding this matter, but as of the time of writing, no response has been received from Reynolds Tobacco regarding this issue. Additionally, the relevant brands mentioned in the above content have either not responded or declined to comment.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com


