The “Starting Point” Enterprise: A Game-Changer in Global New Tobacco
Nicotine, the core raw material in next-generation products (NGPs), is commonly regarded as the supply chain’s “starting point.” Hubei Heno Biological Engineering Co., Ltd. (hereafter “Heno Bio”) holds this pivotal position through its research, development, and production of nicotine, exerting far-reaching influence across the global NGP landscape.
On January 9, 2025, at the 2025 NGP Global Compliance Development New Year Symposium hosted by 2Firsts, Heno Bio and 2Firsts officially announced a strategic alliance focused on compliance—instantly capturing industry-wide attention. The event’s theme, “Compliance Drives Growth,” resonates with the sector’s urgent demand for solid regulatory foundations as NGPs continue to evolve rapidly.
Two key messages emerged from the Symposium:
1. 2025 as the Starting Point: The future trajectory of the industry will hinge on robust compliance measures.
2. Supply Chain Empowerment: By initiating strategic action at the “starting point” of the value chain, Heno Bio aims to cascade compliance benefits across downstream stakeholders.

This partnership not only enhances Heno Bio’s commitment to regulatory standards but also leverages 2Firsts’ role as a leading global media and think tank in the NGP space, amplifying best practices and insights to a worldwide audience.
The World’s First Nicotine Enterprise to Publicly Announce a PMTA-TPMF Application
During the Symposium’s strategic partnership ceremony, Heno Bio General Manager Liu Qizhou delivered a keynote titled “Compliance Best Practices from a Nicotine Enterprise Perspective.”

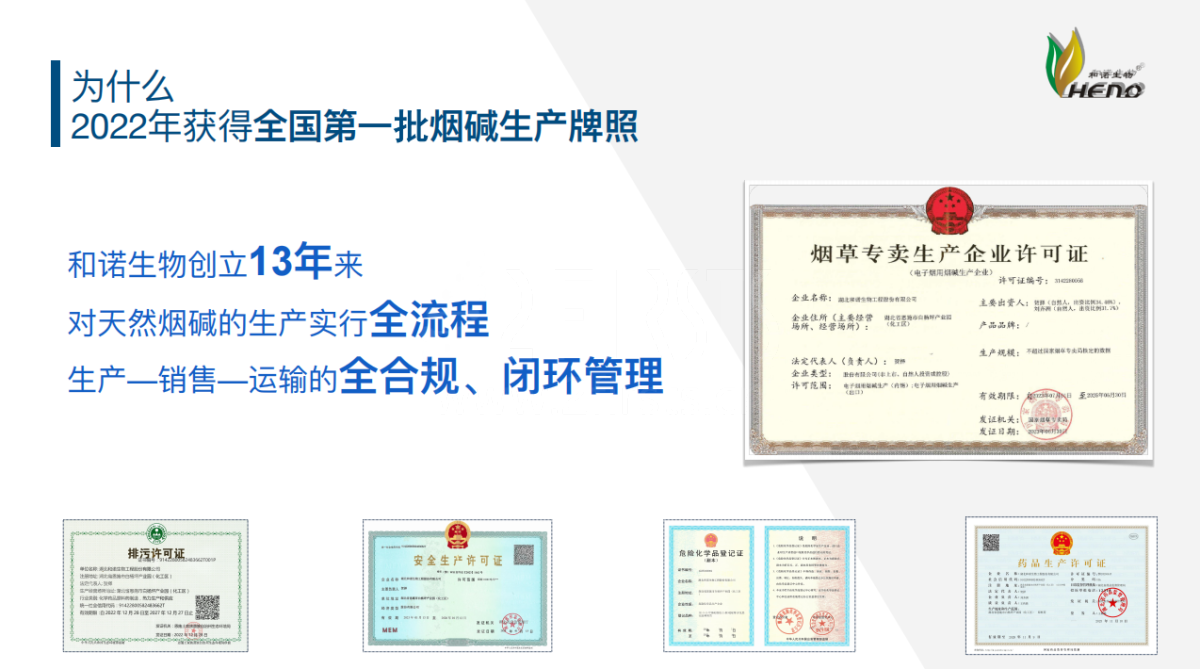
Liu highlighted that Heno Bio was among the first batch of companies in China to secure a Tobacco Monopoly Production Enterprise License in 2022 and successfully renewed it in 2023—reflecting the firm’s longstanding dedication to regulatory adherence. Now, by publicly announcing its PMTA application in the United States, Heno Bio stands out as the world’s first nicotine enterprise to openly take this critical step, reinforcing its image as a forward-looking force in global NGP compliance.
According to 2Firsts COO Echo Guo, Heno Bio’s pioneering stance not only elevates the company’s global competitiveness but also offers valuable compliance solutions to downstream players. It serves as a real-world example of how a supply-chain leader can champion new regulatory ideas and frameworks.
Why PMTA Is the Global “Entry Ticket”
Heno Bio’s compliance strategy centers on obtaining PMTA approval in the United States—the world’s largest e-cigarette market, holding roughly one-third of global market share. Beyond its market size, the U.S. also shapes international trends in both product innovation and regulatory direction. The FDA’s PMTA (Premarket Tobacco Product Applications) system is known for its rigorous and science-based criteria, often dubbed the “gold standard” of the industry.
Since the FDA formalized its PMTA framework in 2021, 34 products have received Marketing Granted Orders (MGOs), primarily from major multinational tobacco corporations. Looking ahead, 2024 marks the opening phase of the FDA’s “5-Year Strategic Plan,” which merges accelerated reviews with more stringent enforcement. By 2025, most e-cigarette and NGP brands seeking entry into the U.S. market will likely face PMTA requirements as a non-negotiable step.
“TPMF + TPMP”: A Two-Pronged Compliance Approach
Although Full PMTA applications are typically filed by brand owners or manufacturers, Heno Bio—positioned at the “starting point” of the supply chain—has proactively pursued compliance through the FDA’s TPMF (Tobacco Product Master Files) and TPMP (Requirements for Tobacco Product Manufacturing Practice).
1. Proactive Self-Regulation
By documenting its processes and adhering to rigorous production standards, Heno Bio demonstrates its scientific and technological strengths in meeting regulatory benchmarks.
2. Downstream Enablement
Once Heno Bio’s nicotine-related master files are approved, downstream brands can reference Heno Bio’s TPMF in their own PMTA submissions (with authorization), eliminating redundant testing and paperwork. This can significantly reduce both compliance costs and time-to-market.
Building a Supply Chain Blueprint for PMTA
How the “Starting Point” Accelerates the Entire Value Chain
As the global new tobacco market continues to expand and regulations tighten, every sector node—from research and development to sales—must integrate compliance measures. The Heno Bio–2Firsts alliance underscores the strategic advantage of initiating compliance at the “starting point,” ultimately benefiting the entire supply chain.
Heno Bio’s fortification of regulatory standards signals a call to action for its upstream suppliers to ensure legal, safe, and high-quality raw material sourcing. This move further reassures downstream stakeholders, offering greater confidence in product safety and regulatory clarity. Such an impact has the potential to transform the market, paving the way for a more stable and sustainable future for NGPs worldwide.
About HENO
Hubei Heno Biological Engineering Co., Ltd. is one of the first batch of natural nicotine production enterprises in the country to obtain the "Tobacco Monopoly Production Enterprise License", and the first domestic enterprise to apply for the raw material production permit with the natural nicotine extraction process route. It has obtained the "Drug Production License" issued by the Hubei Provincial Drug Administration, is a national-level specialized, refined, unique and innovative giant enterprise, a nicotine production enterprise with CANS laboratory nicotine testing capabilities, and a customs advanced certification (AEO) enterprise. The company has drafted the "Production, Processing and Technical Control Specifications for (1-Methyl-2-(3-pyridyl)pyrrolidine)", filling the global standard gap in the production, processing and technical control of natural nicotine. The company has built a safe, environmentally friendly, green and 5G smart park in Baiyangping Industrial Park Chemical Zone in Enshi, Hubei, with an annual capacity to process more than 60,000 tons of tobacco waste and a nicotine production capacity of more than 600 tons.
Company's official website:https://www.henonicotine.com/about-us_d1
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com