It won't be until early November 2023 that cigarette packaging and advertisements will feature graphic health warnings.
The Eastern District Court of Texas in the United States has issued an order to further delay the effective date of the Food and Drug Administration's (FDA) graphic cigarette health warning regulation from October 6, 2023, to November 6, 2023, for an additional 31 days.
According to the National Association of Tobacco Outlets (NATO), a court order issued on November 7 has extended the deadline for manufacturers and retailers to submit their preferred rotation plans for cigarette health warning labels to the FDA by 31 days.
Every tobacco manufacturer and retailer who makes their own cigarette advertisements is required to submit a plan to the FDA that outlines a schedule for rotating the use of 11 graphic cigarette health warnings in their ads. The association stated that the preferred submission deadline for the cigarette health warning rotation plan is now January 6, 2023.
According to NATO, the court is expected to make a ruling on the motion for summary judgment in the case within 31 days.
In March 2020, the FDA released a final rule about warnings that includes text and image combinations that describe some of the health risks associated with smoking. However, implementation has been repeatedly delayed after several tobacco companies requested a specific date be set.
The Eastern District Court of Texas has postponed the deadline for the final time on August 20, setting the new deadline for October 6, 2023.
These warnings must appear on the top 50% of the front and back of cigarette packaging, as well as on at least 20% of the top of cigarette advertisements. Additionally, as previously reported by Convenience Store News, these warnings must be randomly and evenly displayed and distributed on cigarette packaging, and rotated quarterly in cigarette advertisements.
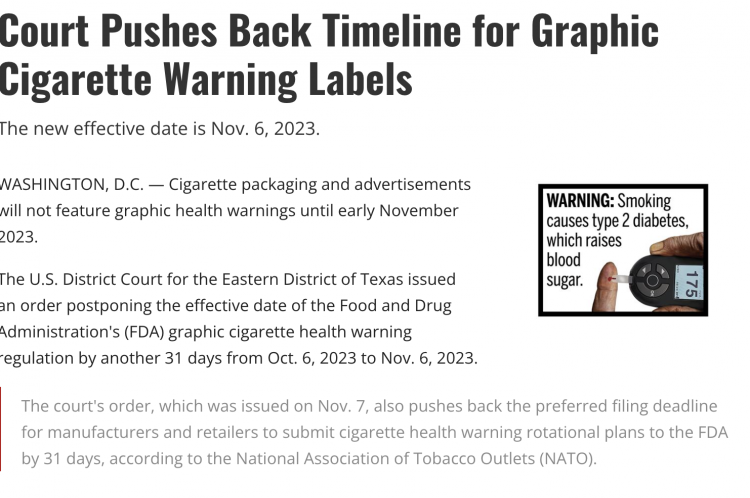
There are 11 essential warnings that need to be addressed. They include:
Warning: Tobacco smoke can harm your children. Warning: Tobacco smoke can cause deadly lung diseases in non-smokers. Warning: Smoking can lead to head and neck cancer. Warning: Smoking can cause bladder cancer, leading to blood in urine. Warning: Smoking during pregnancy can hinder fetal growth. Warning: Smoking can block arteries, leading to heart disease and stroke. Warning: Smoking can cause chronic obstructive pulmonary disease, a potentially fatal lung disease. Warning: Smoking can decrease blood flow and cause erectile dysfunction. Warning: Smoking can decrease blood flow to the limbs, possibly requiring amputation. Warning: Smoking can cause type 2 diabetes, raising blood sugar. Warning: Smoking can cause cataracts, leading to blindness.
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.








