
Special statement:
This article is for internal industry communication only and does not make any recommendations for specific brands or products.
The images presented in this article are only used to describe the facts and are not intended as advertisements for any products.
Minors are prohibited from accessing this article.
Key Takeaways:
Significant Increase in Approvals: The MHRA approved a total of 1,552 registration codes between September 8 and 14, a 32-fold increase from the previous week.
Concentrated Brand Approvals: The list includes registrations from major international brands such as ELFBAR, SMOK, VAPORESSO, and INNOKIN, covering devices, kits, and pods.
Pod Products Dominate: Pod-based products accounted for over 90% of the total approvals this period, with 1,397 registration codes approved. This is approximately a 33-fold increase from the previous week.
Comprehensive Product Coverage: The approvals include a wide range of products, such as rechargeable devices, refillable devices, complete kits, and standalone components.
2Firsts September 16, 2025 - According to the UK's compliance process, e-cigarette products must be listed in the public notice database of the Medicines and Healthcare products Regulatory Agency (MHRA). Once a product is listed, it means its registration code has passed the compliance review and is authorized for legal sale in the UK market.
To help industry professionals understand the approval status of new products in the UK market, 2Firsts regularly compiles and analyzes relevant information from the MHRA notices.
Below is an analysis of the notices updated from September 8 to September 14, covering major brands, product types, and initial market trends.
During this period, the MHRA announced a total of 1,552 registration codes. The brands involved include PIXL, ELFBAR, HAYATI, SMOK, KANGVAPE, JNR, OXVA, VAPORESSO, INNOKIN, and others.
In terms of product volume, the 1,552 registration codes updated between September 8 and September 14 represent a 32-fold increase compared to the 46 codes from the previous week (September 1 to September 7).
ELFBAR, SMOK, KANGVAPE Launch New Devices
In the product category "Electronic cigarette – Rechargeable, placed on the market with one type of e-liquid (fixed combination). Any rechargeable which can also be used as a refillable should be reported under the refillable category," a total of 29 registration codes were updated from September 8 to 14, from the brands OK VAPE and PIXL.
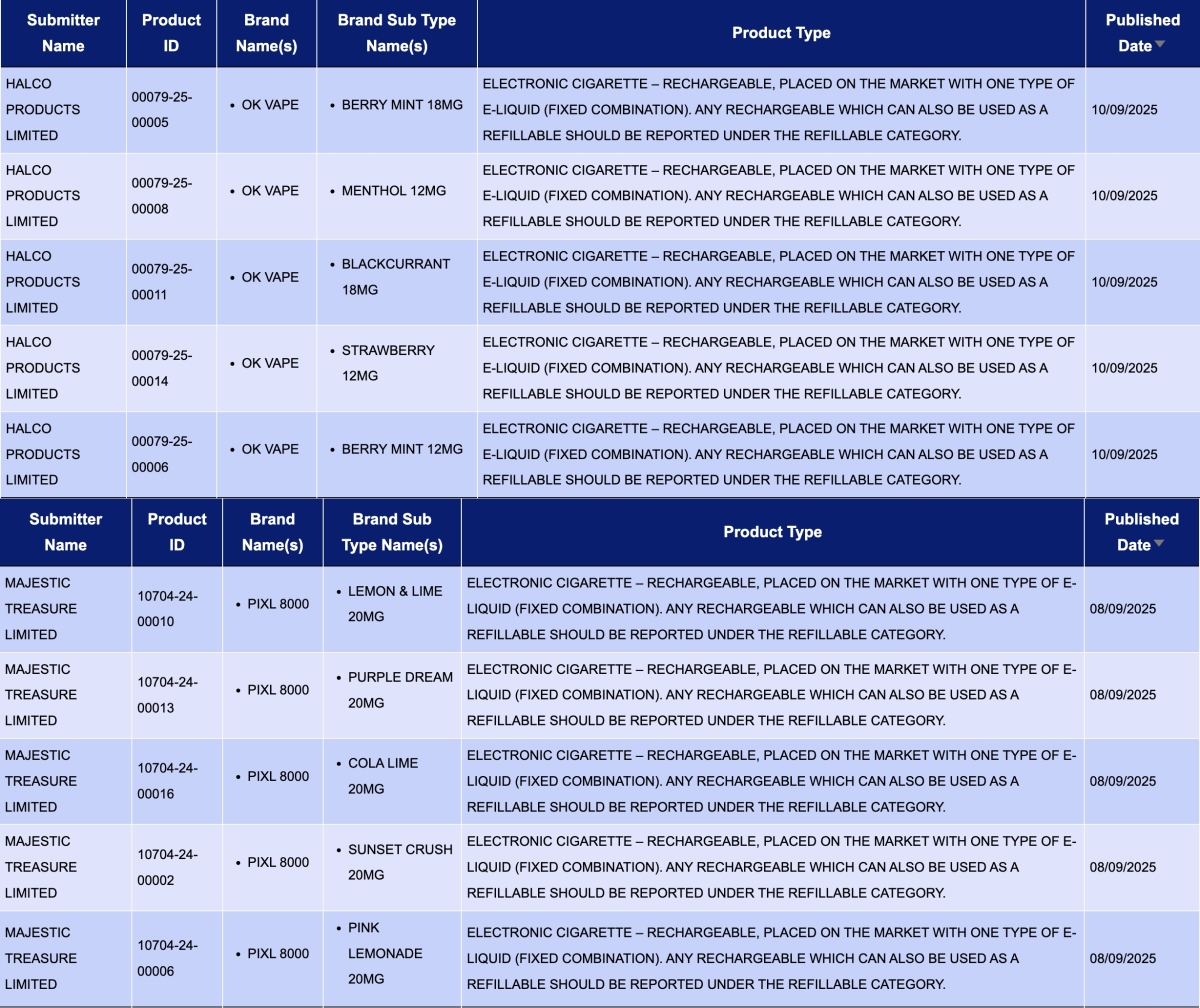
- HALCO PRODUCTS LIMITED, as the applicant, announced 12 registration codes for the OK VAPE brand. The products include flavors like BERRY MINT, MENTHOL, BLACKCURRANT, and STRAWBERRY, with nicotine concentrations of 18MG and 12MG.
- MAJESTIC TREASURE LIMITED, as the applicant, announced 12 registration codes for the PIXL 8000. The products feature flavors such as BLUEBERRY RASPBERRY, FRESH MINT, and KIWI PASSION GUAVA, all with a nicotine concentration of 20MG.
In the category "Electronic cigarette – Rechargeable, device only Any rechargeable which can also be used as a refillable should be reported under the refillable category," a total of 18 registration codes were updated from September 8 to 14, involving brands like ELFBAR, LOST MARY, HAYATI, SMOK, and KANGVAPE.
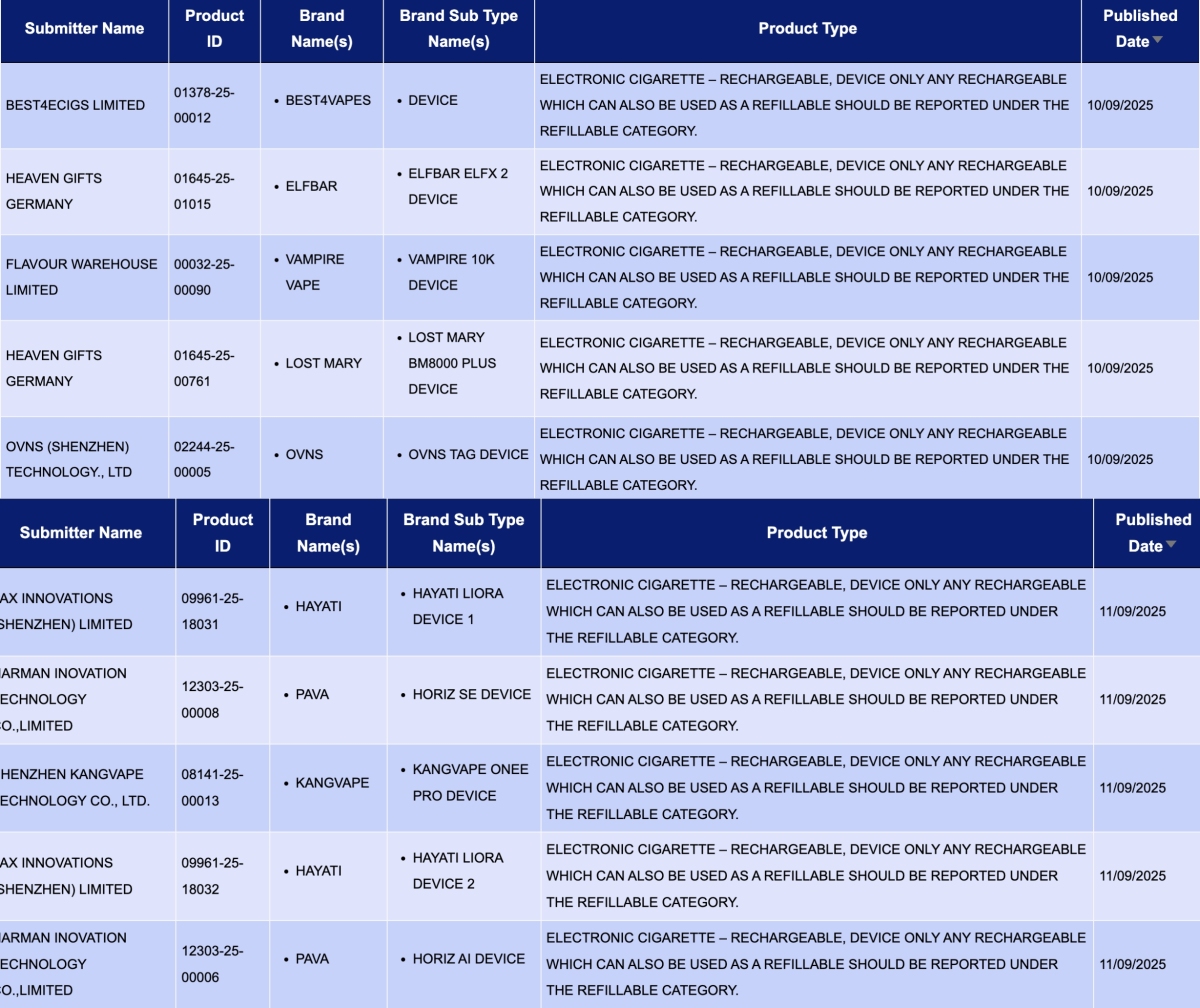
HEAVEN GIFTS GERMANY, as the applicant, announced 3 registration codes for the ELFBAR and LOST MARY brands:
- ELFBAR ELFX 2 Device
- LOST MARY BM8000 PLUS Device
- ELFBAR ELFA Master V2 Rechargeable Device
PAX INNOVATIONS (SHENZHEN) LIMITED, as the applicant, announced 4 registration codes for the HAYATI brand:
- HAYATI LIORA device 2
- HAYATI Mini GO device 1
- HAYATI LIORA device 3
- HAYATI LIORA device 1
IVPS TECHNOLOGY B.V., as the applicant, announced the SPACEMAN 10K Device for the SMOK brand.
SHENZHEN KANGVAPE TECHNOLOGY CO., LTD., as the applicant, announced the KANGVAPE ONEE PRO DEVICE for the KANGVAPE brand.
In the category "Electronic cigarette – Refillable, placed on the market with one type of e-liquid (fixed combination)," a total of 15 registration codes were updated from September 8 to 14, involving the NEXEL and SNOWPLUS brands.
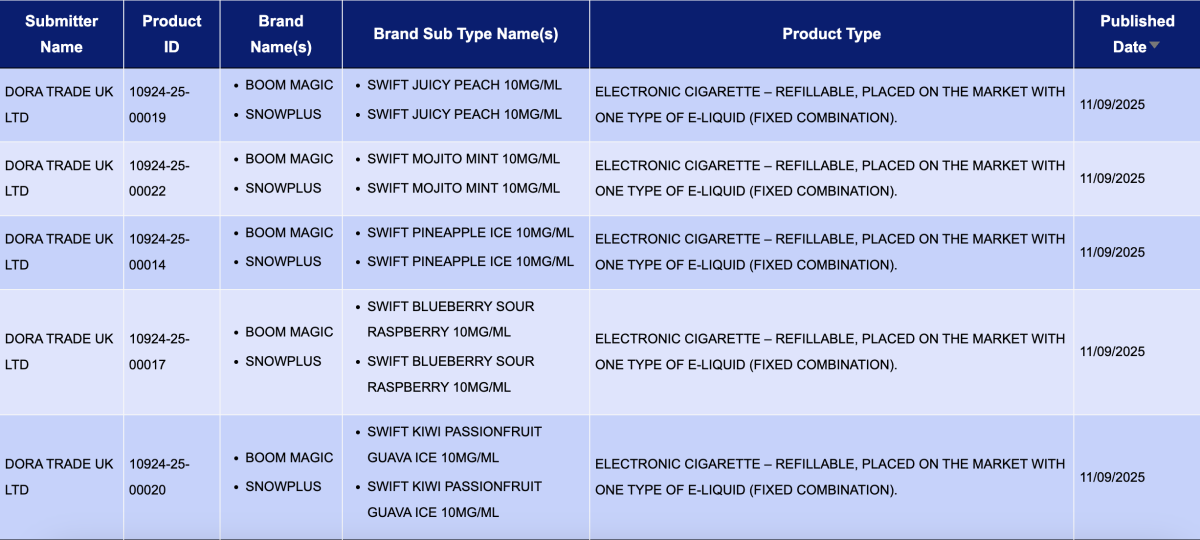
DORA TRADE UK LTD, as the applicant, announced 13 registration codes for the Boom Magic and SnowPlus brands, with all products having a nicotine concentration of 10MG.
NEXGEN TECHNOLOGY HK LIMITED, as the applicant, announced 2 registration codes for the NEXEL brand.
OXVA, INNOKIN Launch New Open Systems
In the category "Electronic cigarette – Refillable, device only," a total of 35 registration codes were updated from September 8 to 14, involving brands like JNR, OXVA, INNOKIN, and VAPORESSO.
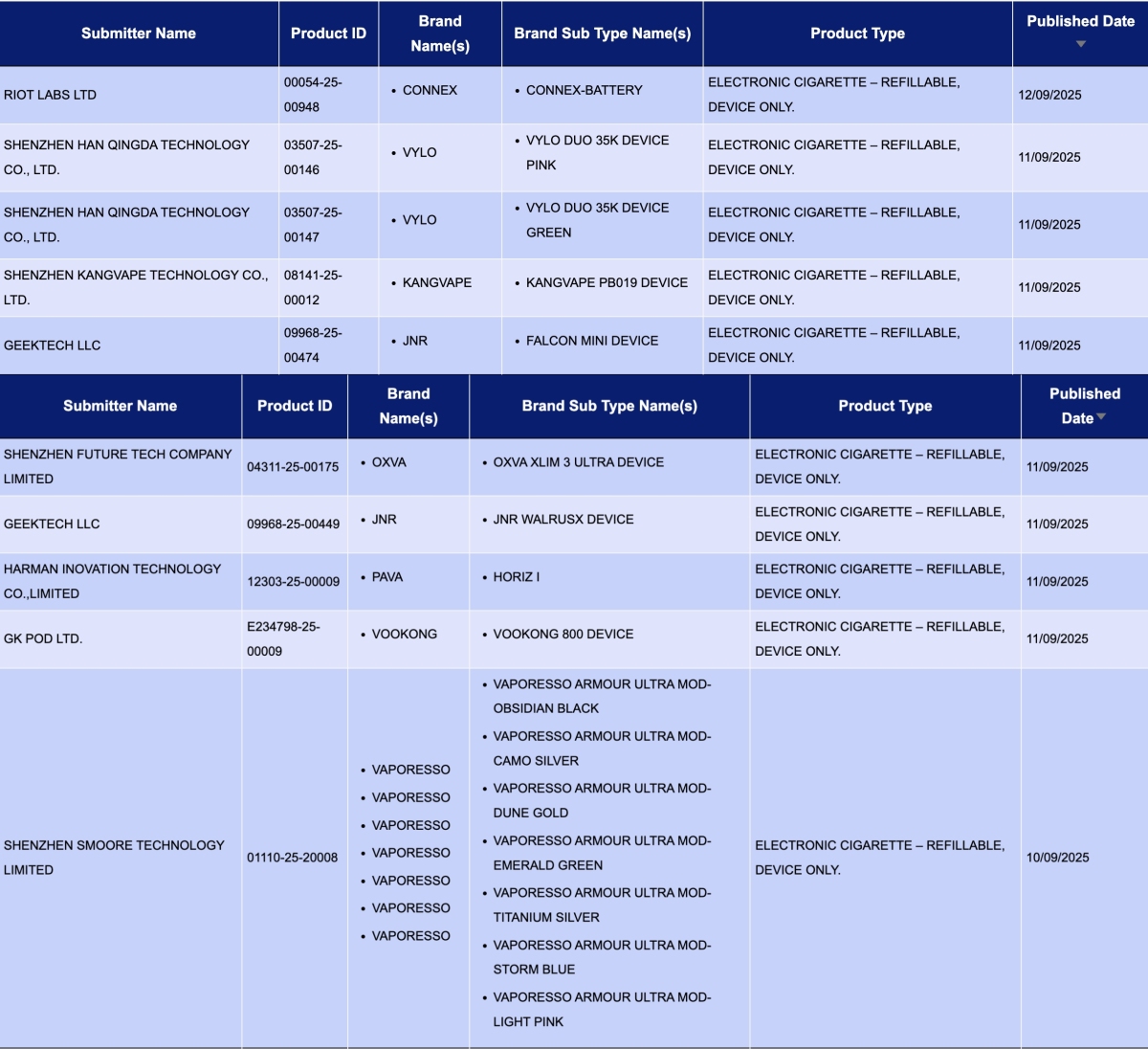
GEEKTECH LLC, as the applicant, announced 3 registration codes for the JNR brand:
- JNR WalrusX Device
- FALCON Mini Device
- FALCON Mini POD
SHENZHEN FUTURE TECH COMPANY LIMITED, as the applicant, announced 4 registration codes for the OXVA brand:
- OXVA XLIM PRO 3 Device
- OXVA XLIM 3 ULTRA Device
- OXVA NeXLIM GO Device
- OXVA XLIM GO 2 Device
SHENZHEN INNOKIN TECHNOLOGY COMPANY LIMITED, as the applicant, announced 2 registration codes for the INNOKIN brand: PLEXUS PRO Kit and Sensis Neo kit.
SHENZHEN SMOORE TECHNOLOGY LIMITED, as the applicant, announced 7 colors for the VAPORESSO ARMOUR ULTRA MOD for the VAPORESSO brand.
In the category "Kit – Pack containing more than one different e-cigarette device and/or more than one different refill container/cartridge," a total of 5 registration codes were updated from September 8 to 14, involving brands like SMOK, VAPORESSO, and LOST VAPE.
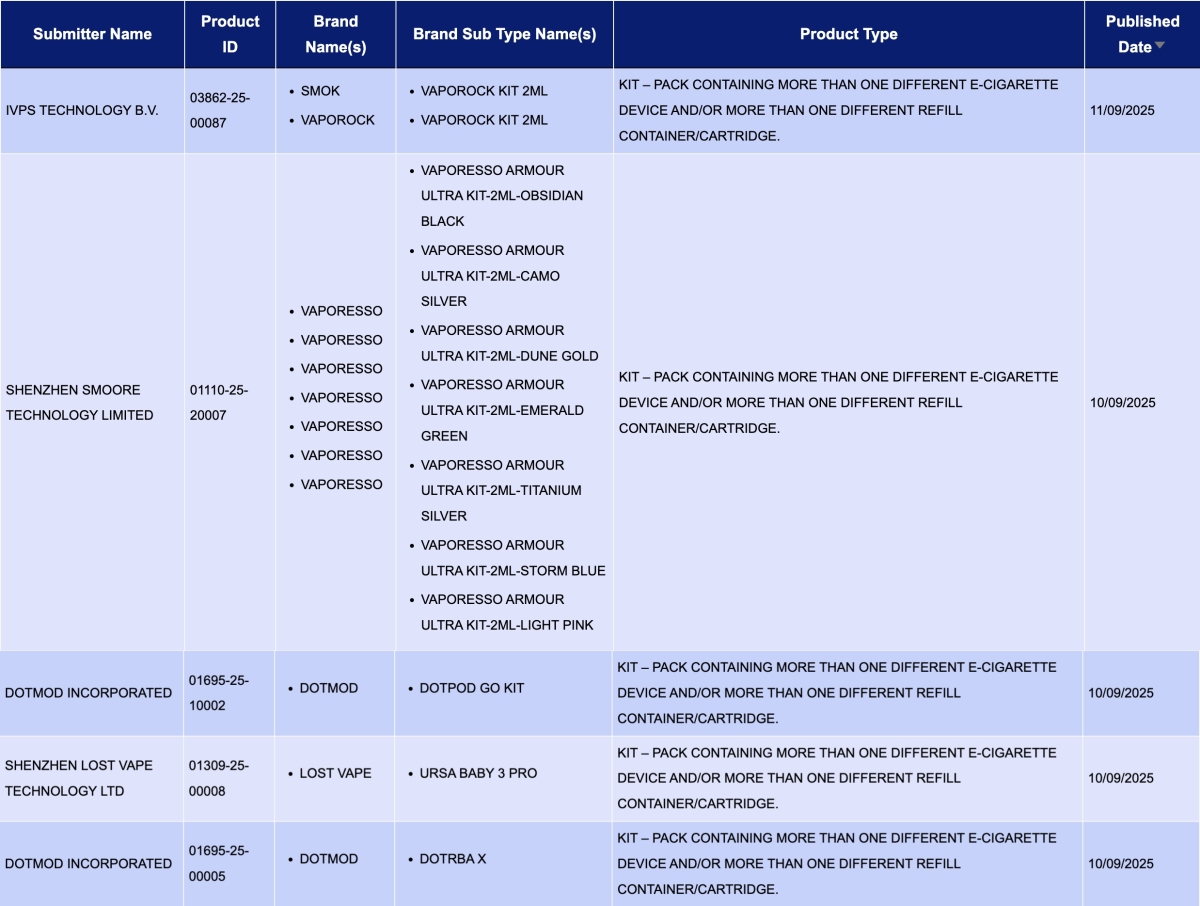
IVPS TECHNOLOGY B.V., as the applicant, announced the VAPOROCK KIT 2ml for the SMOK and VAPOROCK brands.
SHENZHEN SMOORE TECHNOLOGY LIMITED, as the applicant, announced 7 colors for the VAPORESSO ARMOUR ULTRA KIT for the VAPORESSO brand.
SHENZHEN LOST VAPE TECHNOLOGY LTD, as the applicant, announced the URSA BABY 3 PRO for the LOST VAPE brand.
1,397 Pods Approved, a 33-Fold Increase
In the category "Individual part of electronic cigarette capable of containing e-liquid," a total of 52 registration codes were updated from September 8 to 14, involving brands such as SMOK, VAPOROCK, KAN GVAPE, HAYATI, PAVA, SKE, and INNOKIN.
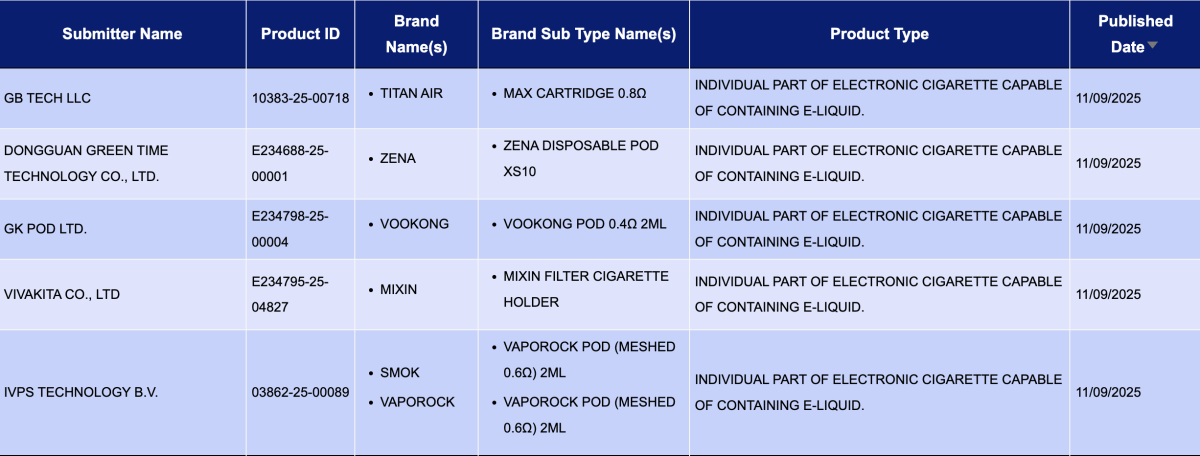
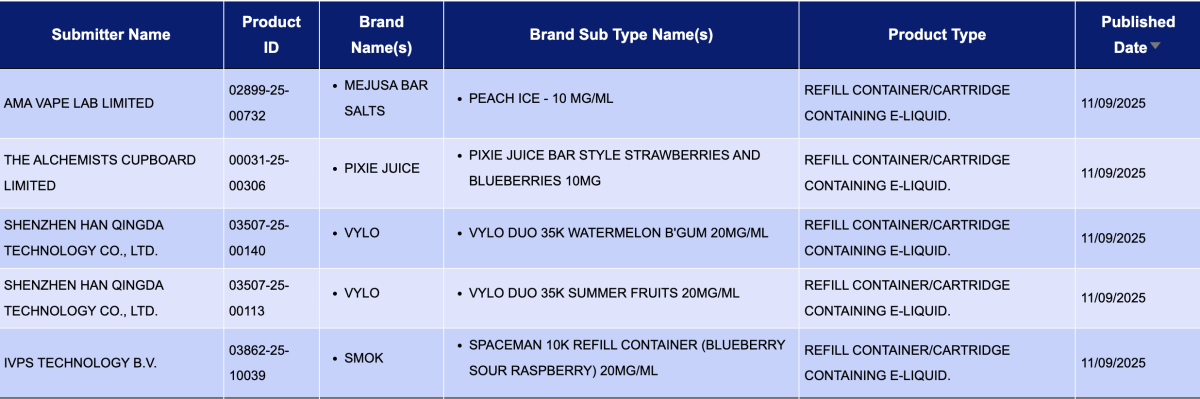
In the category "Refill container/cartridge containing e-liquid," a total of 1,397 registration codes were updated from September 8 to 14, involving brands like INNOKIN, SMOK, SKE, HAYATI, and FUMOT. Compared to the 40 pods announced the previous week (September 1 to 7), the number of pod approvals this week saw a substantial increase, approximately 33 times higher.
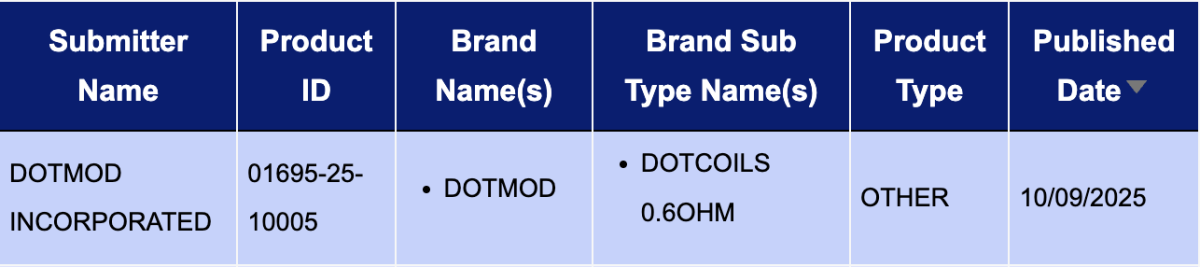
In the "Other" category, a single registration code was updated from September 8 to 14: the dotCoils 0.6ohm from DOTMOD INCORPORATED.
As a leading global NGP media and think tank, 2Firsts is dedicated to providing the latest product and technological information and insights to practitioners around the world in various categories such as e-cigarettes, heated tobacco products, and modern oral products. It aims to drive technological changes and innovations in NGP products worldwide, thereby offering tobacco consumers globally with safer products and lifestyles.
With a source of information covering the supply chains in China and global markets, 2Firsts product coverage has become one of the most influential platforms for new product and technology releases globally.
Contact 2Firsts for the following services:
1. Providing leads on new products and technologies;
2. Offering comments on products and technologies;
3. Seeking media coverage for your products;
4. Identifying sales channels for products.
Contact Information: Email: info@2firsts.com
Contact CEO Alan Zhao of 2Firsts on LinkedIn.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







