Special announcement:
1.This article is intended for internal industry communication only and does not make any recommendations for brands or products.
2.The images displayed in this article are used solely for factual description and not intended as any form of product advertisement.
3.This article is not intended for underage individuals.
Key Points:
·32 new SKUs announced: The e-cigarette brand VEEV under Philip Morris International (PMI) has successfully completed the notification process with the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK and obtained market approval, in compliance with local regulatory requirements.
·30 flavors to choose from: VEEV brand reveals 30 pod SKUs, covering flavors such as fruit, mint, and tobacco, with nicotine concentrations of 1.8% and 1.6%.
·Eco-friendly recycling: VEEV brand claims that both their devices and pods can be recycled, and returning any e-cigarette product for recycling can get you a £5 voucher.
·Charging time of 45 minutes: The VEEV ONE and VEEV ONE SE utilize ceramic heating technology, allowing for a quick charging time of just 45 minutes.
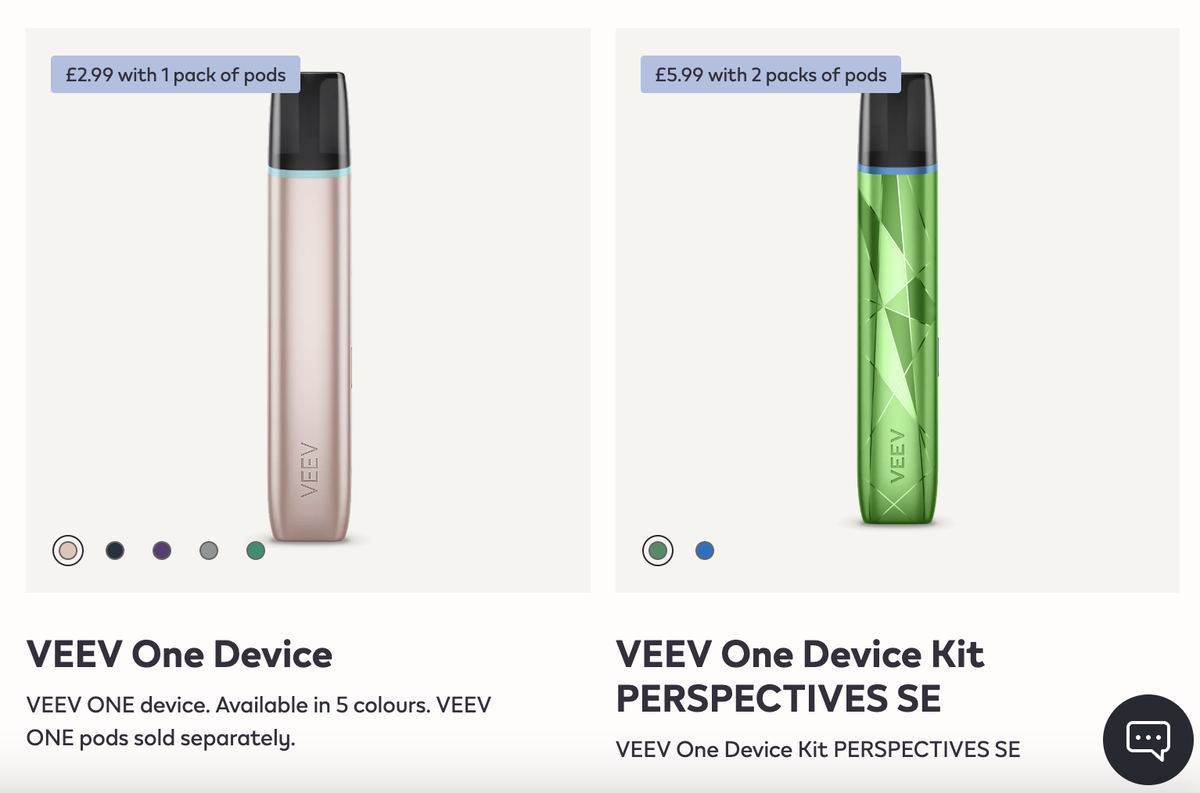
·Launched in the UK market: The VEEV ONE product series has landed on the IQOS official website, and is simultaneously available on e-cigarette distributor websites such as The Electric Tobacconist, Vape UK, and Sainsburys targeting the UK region.
【By 2Firsts】Recently, an investigation of the UK Medicines and Healthcare products Regulatory Agency (MHRA) e-cigarette product notification database revealed that Philip Morris International (PMI) has announced 32 new SKUs for its e-cigarette brand VEEV in early May of this year. These new SKUs have completed the notification process with the MHRA, indicating they have been granted marketing authorization and are now officially on the market in the UK for sale.
A search of IQOS’s official UK website by 2Firsts shows that the VEEV NOW disposable e-cigarette is no longer for sale. Meanwhile, the VEEV ONE device and pod series have been officially launched.
VEEV Unveils 32 SKUs of Devices and Pods
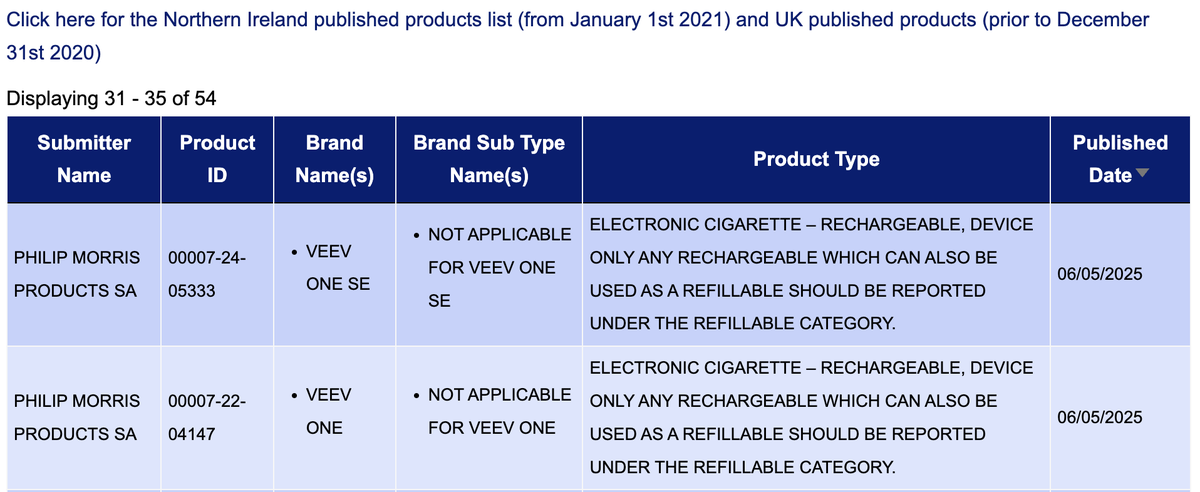
2Firsts has collected and compiled detailed information on e-cigarette product notifications from VEEV in early May in the UK Medicines and Healthcare products Regulatory Agency (MHRA) database.
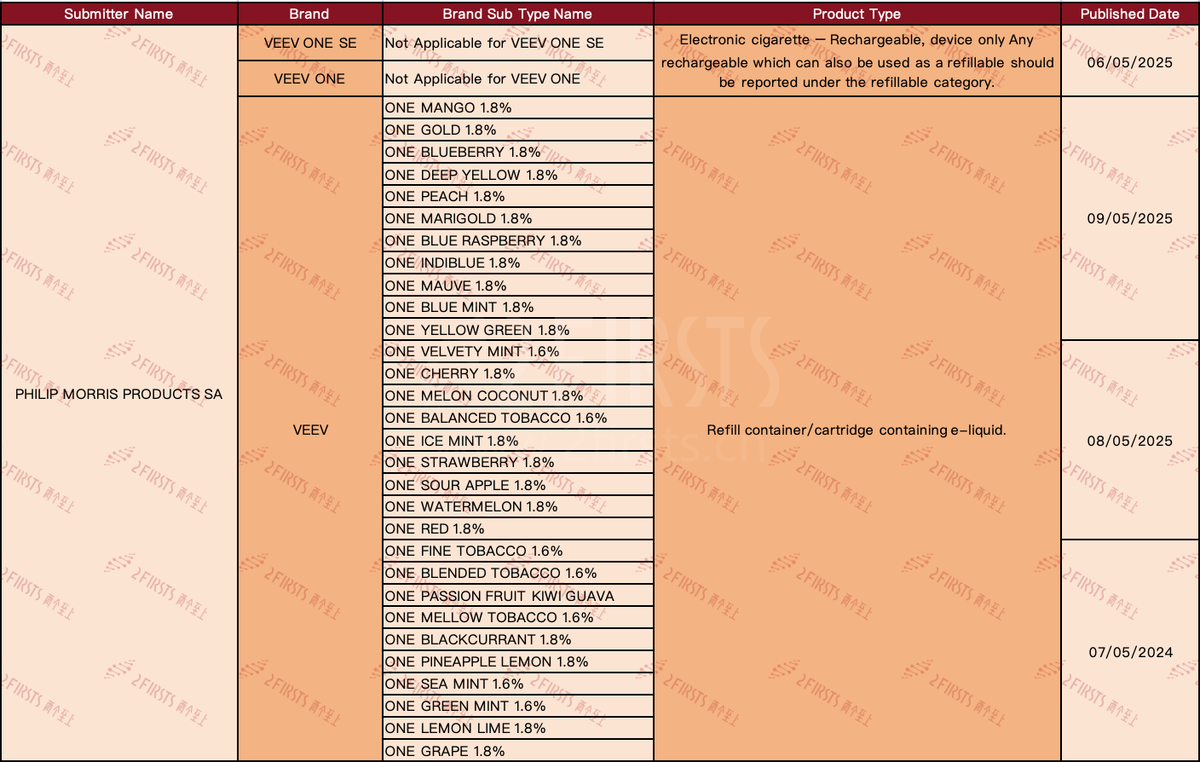
According to public information, in early May, VEEV published 32 SKUs in the Medicines and Healthcare products Regulatory Agency (MHRA) e-cigarette query system in the UK. These new SKUs were all applied for registration by PHILIP MORRIS PRODUCTS SA, specifically for the VEEV ONE SE, VEEV ONE, and VEEV brands.
VEEV ONE SE & VEEV ONE are Pod System Main Devices
VEEV ONE SE and VEEV ONE are two e-cigarette device SKUs categorized as "rechargeable e-cigarettes, device only", indicating that any rechargeable and refillable device should be classified as refillable category. This means that these are pod system main devices, rather than e-liquid (or pod) subtypes with specific flavors or nicotine content.
VEEV Offers 30 Pod Flavors With 1.6% and 1.8% Nicotine Options
·The remaining 30 SKUs under the VEEV brand are "supplement containers/pods containing e-liquid," indicating that they are e-liquid refills or pre-filled pods for e-cigarette devices.
·The VEEVpod comes in a variety of flavors, including fruit flavors, mint/cool flavors, and tobacco flavors.
·The majority of VEEVpod flavors contain 1.8% nicotine, including tobacco and mint flavors such as VELVETY MINT, BALANCED TOBACCO, FINE TOBACCO, BLENDED TOBACCO, MELLOW TOBACCO, SEA MINT, GREEN MINT. The nicotine concentration of these flavors is 1.6%.
VEEV ONE Kit With Two Pods Enters UK Market
2Firsts collected the parameter information of the VEEV ONE device and pod series, as shown in the following figure:
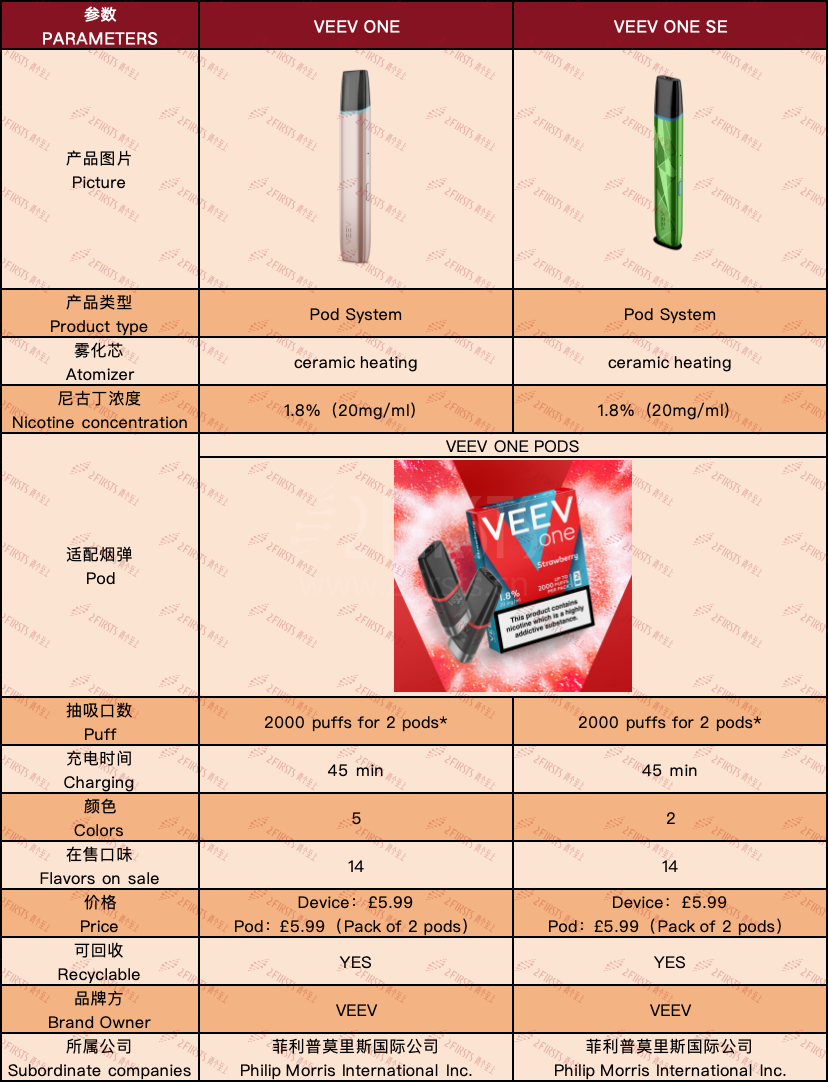
According to the specifications, the VEEV ONE kit has a capacity of 2,000 puffs, both the VEEV ONE and VEEV ONE SE use ceramic heating, and the charging time is 45 minutes. The brand claims that both the device and pods can be recycled. The official website states that "recycling any e-cigarette product will earn you a £5 voucher.

The VEEV ONE series products are all sold on the IQOS official website. Currently, the website displays 14 pod flavors to choose from, mainly covering fruits, mint, and classic tobacco flavors. All pods have a nicotine content of 1.8% (equivalent to 20mg/ml).
Currently, the VEEV ONE series of products has been launched on the IQOS official website and is also available on e-cigarette distributors' websites focused on the UK market such as The Electric Tobacconist, Vape UK, and Sainsburys. It is reported that the series was first released in September 2024.
The VEEV brand was last publicly listed with the UK Medicines and Healthcare products Regulatory Agency (MHRA) in May 2024, with the next listing scheduled for May 6th to May 9th, 2025.
The UK government is set to implement a ban on disposable e-cigarettes starting June 1, 2025. The ban will prohibit the sale, supply, and possession of disposable e-cigarette products, including those containing nicotine and those without nicotine. However, reusable and refillable e-cigarette products will continue to be legally sold.
As a leading global NGP media and think tank, 2Firsts is committed to providing the latest product and technology information and insights for practitioners worldwide in various categories such as e-cigarettes, heated tobacco products, and modern oral products. We aim to drive technological transformation and innovation in global NGP products, ultimately bringing consumers a safer product and lifestyle.
With information sources covering the supply chain in China and global markets, 2Firsts' product reports have become one of the most influential platforms for new product and technology releases globally.
Welcome to Contact 2Firsts:
1. Providing leads on new products and technologies;
2. Offering commentary on products and technologies;
3. Seeking media coverage for your products;
4. Researching sales channels for products;
······
Contact Information:
1.Email: info@2firsts.com
2.Contact CEO Alan Zhao of 2Firsts on LinkedIn.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







