PMI is often asked if it truly intends to end cigarette sales.
The answer is: yes.
Philip Morris International acknowledges that the decline in cigarette sales - or broadly speaking, the decline in the combustible product portfolio of PMI - is a necessary component in achieving its ambitions for a smoke-free future.
Fimo International is rapidly making progress towards this goal.
According to data publicly released by Fumo International, their cigarette sales have been on a downward trend for the past decade.
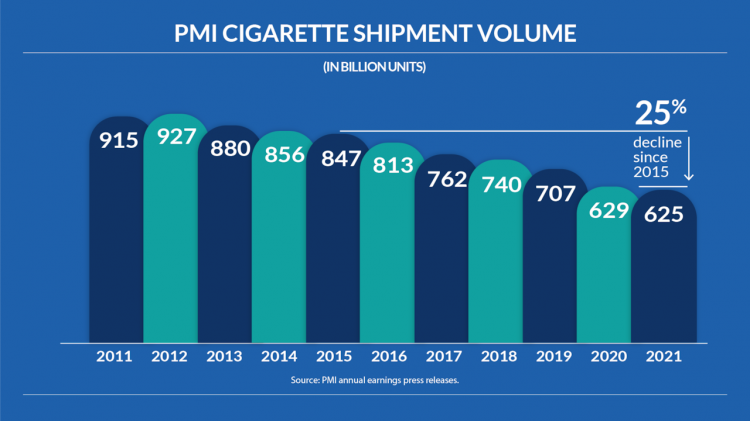
The annual sales volume of cigarettes has declined from 91.5 billion units in 2011 to 62.5 billion units in 2021, decreasing every year over the past nine years.
Furthermore, PMI has set a target for the total shipment of combustible products to be below 550 billion units by 2025, as stated in their 2021 comprehensive report.
The cigarette shipments of PMI (Phillip Morris International) have decreased from 915 billion in 2011 to 625 billion in 2021.
Phimo International aims to ultimately replace cigarettes with smoke-free alternatives.
Since 2008, Philip Morris International has invested over 9 billion dollars in the research and development, product development, production capabilities, scientific validation, and understanding of adult smokers for these innovative products.
This is a successful investment as an increasing number of adult smokers are switching to FiMO International's heated tobacco products, leading to a reduction in cigarette transportation volume.
As of June 30th, 2022, approximately 13.2 million adult smokers have switched to smoke-free alternatives and quit smoking.
The shipment volume of PhiMo International's heated tobacco devices has increased from 7 billion in 2016 to 95 billion in 2021.
These figures are transparently released annually by Fimo International for anyone to access.
Although the optimal choice for any smoker is to completely quit smoking and nicotine, scientifically proven smokeless products are a better option for adult smokers who would otherwise continue smoking.
According to Moira Gilchrist, VP of Strategic and Scientific Communication at Philip Morris International, the company's decision to unilaterally stop selling cigarettes tomorrow will not change anything. "Because it doesn't eliminate the demand for cigarettes. The demand will remain, and others will simply fill it, whether it be competitors or participants in the black market.
Our transition is more significant because we are striving to reduce the long-term demand for cigarettes, ensuring that the decline in the number of smokers continues - and may even accelerate further.
These numbers cannot be ignored," said Stefano Volpetti, President of the PMI Smoke-Free Products category and Chief Consumer Officer. "They clearly depict the transformation that Philip Morris International is driving - first, providing a range of better alternatives for adult smokers and secondly, accelerating smoking cessation.
Our scientific evidence confirms that PMI's smoke-free products, while not without risk, are a better choice than cigarettes for adults who would otherwise continue to smoke.
With support from the industry, stakeholders, and civil society, we believe that cigarette sales can come to an end in many countries within 10 to 15 years. This would be an enduring achievement for the global population of 1 billion smokers, global public health, and our company.
As of June 30, 2022, Philip Morris International (PMI) estimates that approximately 13.2 million adult individuals worldwide (excluding Russia and Ukraine) have switched to their heated tobacco products and quit smoking. These user statistics are reflective of PMI's estimates, which are based on consumer statements and sample-based statistical evaluations, with an average margin of error of +/-5% within the 95% confidence interval in key quantity markets. The accuracy and reliability of user statistics may vary depending on the maturity of individual markets and the availability of information.
Statement:
The content of this article is compiled from third-party information and is intended for industry exchange and learning purposes only.
This article does not represent the views of 2FIRSTS and 2FIRSTS is unable to confirm the authenticity and accuracy of the content. The translation of this article is for industry exchange and research purposes only.
Due to the limitations of the translator's skills, the translated article may not fully reflect the original text. Please refer to the original article for accuracy.
2FIRSTS’ viewpoints and statements on any domestic, Hong Kong, Macau, Taiwan, or international issues are entirely aligned with those of the Chinese government.
The copyright of compiled information belongs to the original media outlet and author. If there is any infringement, please contact us to request removal.
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.








