
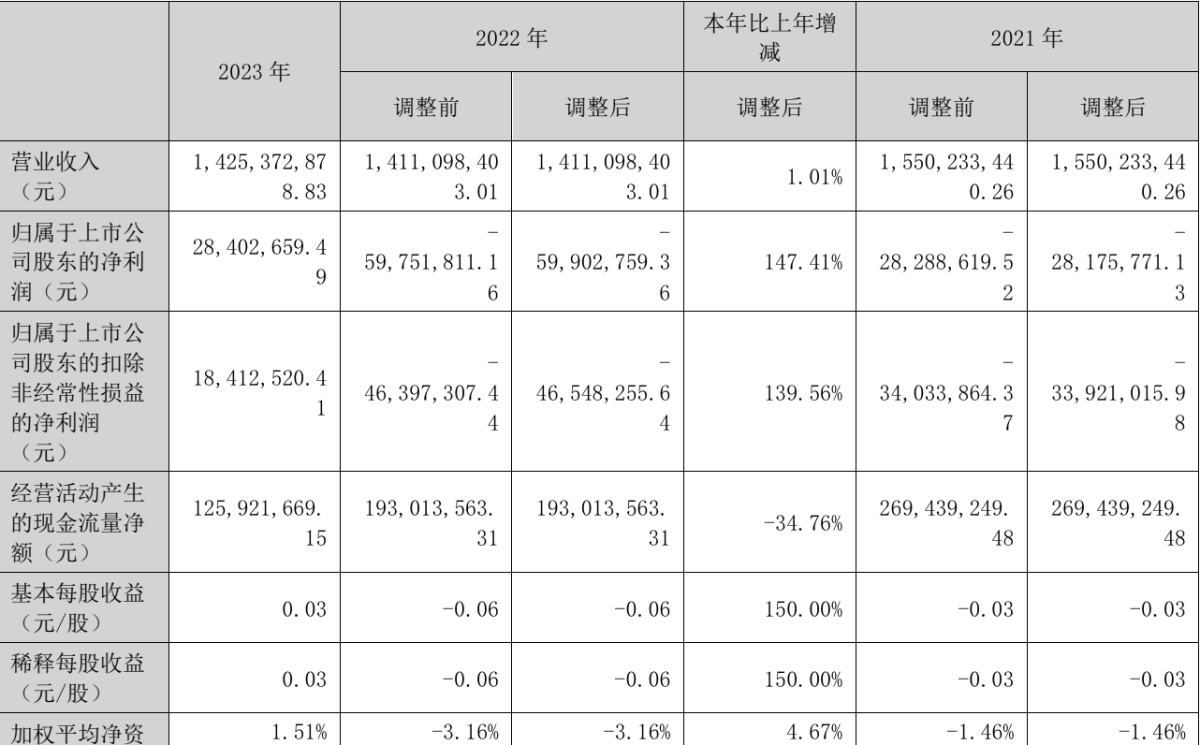
On April 30th, Shunho Co., Ltd. (SZ002565) released its annual performance report, stating that its operating income in 2023 was approximately 1.425 billion yuan, an increase of 1.01% year-on-year. The net profit attributable to shareholders of the listed company was approximately 28.4 million yuan, an increase of 147.41% year-on-year.
Shunho Group has announced that its subsidiary Shunho Yilong holds the rights for the pod brand (domestic sales), e-cigarette accessories brand (domestic sales), and the combination of pod and smoking accessories brand sales (domestic sales). The brand is named "Yilong". During the reporting period, the "Yilong" brand e-cigarette products from Shunho Yilong have successfully launched in some retail stores in Beijing, Shenzhen, and Shanghai. The company plans to continue promoting the "Yilong" products in more regions in the future.
As of now, the subsidiary Green New Peak has obtained the Tobacco Wholesale Production Enterprise License for the e-cigarette processing enterprise. The participating company Mei Zhong Lian has obtained the Tobacco Wholesale Production Enterprise License for the e-cigarette production enterprise. The company's subsidiary Shunho Yilong has obtained the Tobacco Wholesale Production Enterprise License for the e-cigarette brand holding enterprise for domestic sales.
Shunho Corporation stated that, while ensuring the steady development of its environmentally friendly packaging new materials and printing businesses, the company is actively researching and developing new types of tobacco compliance products in accordance with policy requirements. It is also concentrating resources on applying for e-cigarette licenses and continuously conducting research and development investments in industrial hemp new technologies, new products, and overall plant applications. This has enabled the company to transition from a single manufacturing industry to a diversified, multi-industry chain expansion, achieving a new development pattern.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








