
Special Note:
This article is for internal industry communication only and does not make any recommendations for brands or products. The images presented in this article are only used to describe facts and are not intended as product advertisements. This article is not intended for minors to access.
Sikary's "American Dream
On September 24, Yinghe Technology Co., Ltd. in Shenzhen participated in the 2024 New Energy Industry Roadshow event held by the Shenzhen Stock Exchange's "Interactive Easy" platform. When asked about the expansion of its subsidiary and Sikary in the United States and other emerging markets, Yinghe Technology's secretary of the board, Li Chunhui, responded: "Recently, Sikary has had two products pass the first stage audit of the PMTA and obtained an STN number, which helps Sikary expand in the U.S. market.

On a mutual fund stock trading platform, a netizen interpreted it as "Sikary has obtained PMTA authorization for 2 products," sparking industry attention.
What does obtaining an STN number mean for Sikary? What is the driving force behind Sikary's push for PMTA product review? 2Firsts consulted with industry experts to interpret Sikary's new developments in emerging markets.
Obtaining an STN number does not mean passing PMTA
Obtaining an STN number only confirms that a PMTA application has been submitted, but does not prove that the product has passed PMTA review, said Zheng Zhi, PMTA certification consultant and founder of Ziyuan Technology, to 2Firsts.
The full name of PMTA is Premarket Tobacco Application. It refers to the legal requirement for any new tobacco product introduced after February 15, 2007 to undergo approval from the FDA in the United States. The agency thoroughly evaluates whether the product is beneficial for public health before allowing it to be marketed.

According to the FDA official website, the PMTA review process is divided into six stages.
- Presubmission Meetings
- Acceptance Review
- Filing Review
- Application Review
- Action
- Postmarket Requirements
The full name of STN is Submission Tracking Number, which stands for "submission tracking number." It is a code assigned by the FDA system after receiving the applicant's information and conducting a preliminary review, used to identify and match the information submitted by the applicant. This typically occurs before and after the "pre-submission meeting," marking the beginning of the PMTA review process.
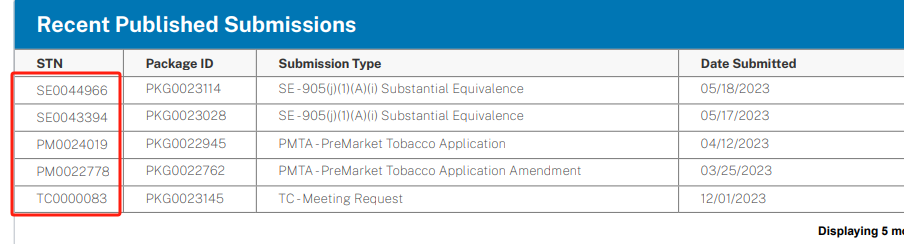
"STN number is just a tracking code automatically assigned by the FDA after the manufacturer submits the application materials to the designated file reception port. It only proves that the product has been submitted for PMTA application, and does not mean the application has been approved. As the PMTA review process progresses, the STN number may be revoked at any time," Zheng explained.
Can an application be resubmitted for review with the same applicant and product after the STN number is rejected?
Zheng Zhi explained that once the STN number is revoked, the same product and applicant cannot reapply for PMTA review within six months.
Due to the high frequency of product updates in the American e-cigarette market, the typical market lifecycle for popular e-cigarette products is about 3 months. Brands or manufacturers will often choose to release upgraded versions of their e-cigarette products at regular intervals.
"Even though the applicant can resubmit the materials for the product, considering the sales prospects in the market, it is highly unlikely that they will submit again," Zheng Zhi said.
Products with STN numbers can enter the United States in the short term
Specifically, for Sikary, having an STN number means that the products they have applied for can legally enter the US market. However, if the STN number is revoked, these products lose the opportunity to enter the US market.
Zheng Zhi stated that due to the large number of projects currently applying for PMTA review, the FDA's capacity for review is limited. Therefore, typically, as long as the application is submitted with the required information, the STN number can be obtained.
"Furthermore, due to the long PMTA review process, legal sales in the United States will not be disrupted in the short term. However, it is not ruled out that at a certain point, the FDA may enforce regulations requiring only products that have passed PMTA approval to be considered legal for sale," Zheng stated.
Zheng Zhi revealed that after obtaining the STN number, the company still needs to wait for FDA approval. During this process, the FDA will review the submitted materials and decide whether to proceed to the next stage.
For example, the need to submit appropriate supplementary evidence requires an investment ranging from tens of thousands to hundreds of millions of dollars. Faced with e-cigarette products that have not yet been market validated, many brand owners are unwilling to bear these costs, which also hinders subsequent reviews from taking place," Zheng Zhi said.
Driving force: UK market faces challenges, actively seeking to capture US market
Facing the financial investment required after PMTA approval, why is Sikary, which originally focused on the UK market, determined to enter the US market?
An observer of the US market known as SKY (pseudonym) engaged in a discussion to analyze Sikary's market trends.
Sikary has been steadily developing in the UK market in recent years, particularly with the introduction of the SKE Crystal Bar series, which has gained significant attention in the market and became a "dark horse" in the capital market last year.

According to SKY, the growth of e-cigarette consumption in the UK, as a mature market, is expected to plateau. Faced with a constant influx of new brands, companies are forced to develop products for other regions in order to ease pressure on the production chain caused by excess stock in various channels.
In January of this year, the British government announced a ban on disposable e-cigarettes, further fueling Sikary's expansion into other regions worldwide.
For example, at the recent Malaysia ANTY exhibition, 2Firsts noted that the SKE brand has started entering the local market this year and sees localizing flavors as the first step.
At the same time, with the increasingly tightened regulations on e-cigarettes in countries around the world, Sikary must speed up its layout to ensure the brand's leading position in these markets," SKY said.
Yinghe Technology also stated that "Sikary has expanded its sales team this year and is gradually expanding its sales area in North America and Southeast Asia through measures such as advertising, social media interaction, grassroots promotions, and large-scale exhibitions."
In addition, the United States, as the world's largest single e-cigarette consumer, saw e-cigarette exports from China to the U.S. reach 18.14 billion U.S. dollars in the first half of the year, representing a year-on-year increase of over 20%.
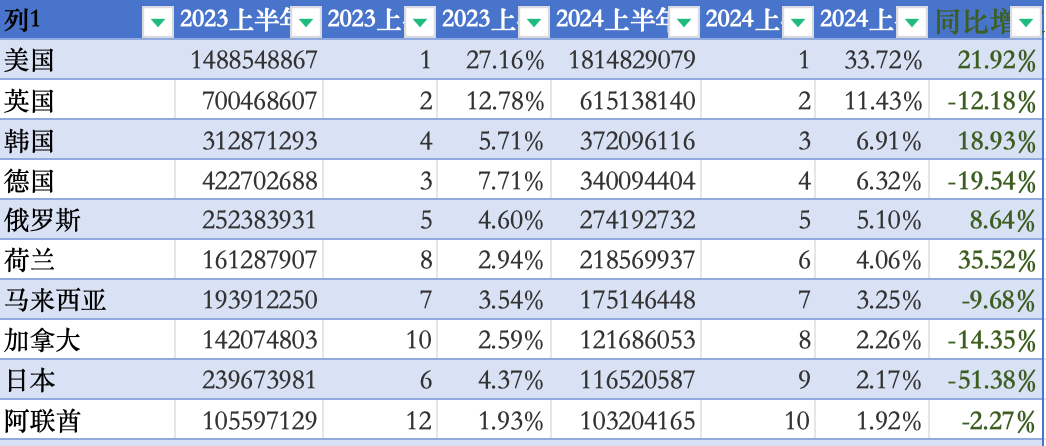
Therefore, it is particularly important for Sikary to enter the American market, despite the fierce competition and strict regulations. The country's high consumer power and acceptance of new products continue to attract the attention of numerous e-cigarette brands worldwide.
SKY pointed out that Sikary's entry into the US market was not a hasty decision, but rather based on various considerations and strategic planning.
As a subsidiary of the publicly listed company Yinghe Technology (300457), it is required to ensure compliance. PMTA certification is a necessary step for tobacco products to enter the US market in compliance, but it is also a costly and complex process. Many small and medium-sized e-cigarette brands have had to give up entering the US market due to their inability to afford the high certification fees.
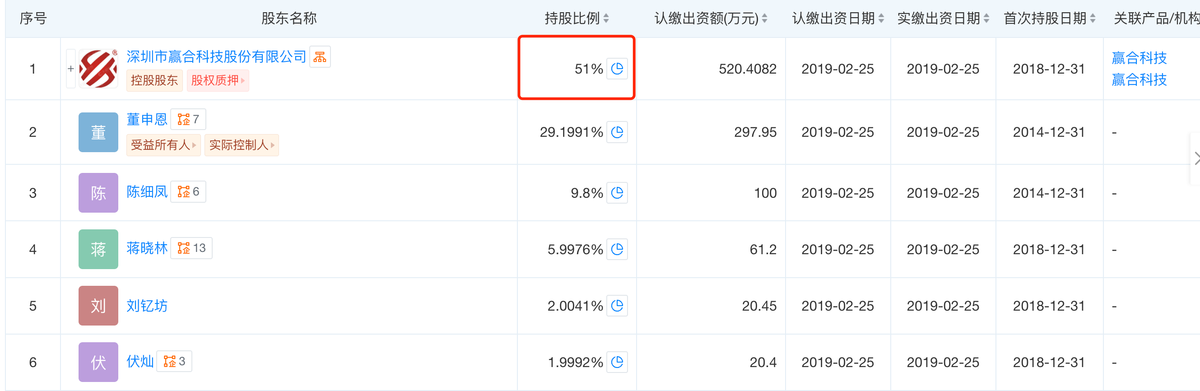
"As a subsidiary of a publicly-listed company, it can afford to invest in the PMTA process for compliance and risk mitigation," said SKY. He believes that the biggest obstacle in the future may be the gradual advancement of the PMTA review process, which will scrutinize product experimental data and the assessment of manufacturing facilities.
"The products that have passed the PMTA have all undergone strict FDA audits of the contract manufacturers, and there are only a handful of companies in China that meet the qualification standards for these audits," said SKY.
Zheng Zhi stated that, to his knowledge, the only contract manufacturers in China that have passed the FDA's "factory inspection" are SMOORE International and Heyuan Group.
Quietly Positioning: Large-Puff Disposable Products Have Landed in the US
Although it is still unclear which of Scour's products have obtained STN numbers, all of Scour's brands have been preparing ahead of time for a "head start."
2Firsts has learned that brands under Scour, such as SKE, Memers, and Vfly, have already appeared in online e-cigarette retail stores in the United States, and all are large-puff disposable products with more than 20,000 puffs.
For example, SKE's large-puff product SKE MG has been listed on the well-known American e-cigarette retail website vape sourcing, priced at $16.99. The product comes pre-filled with 22 milliliters of e-liquid (with a nicotine concentration of 5%), which can be used for 25,000 puffs, with an 850mAh rechargeable battery, and supports three unique e-cigarette modes, including soft mode, normal mode, and boost mode.
It is reported that the product was launched in May of this year through SKE's official US regional X (formerly Twitter) account.
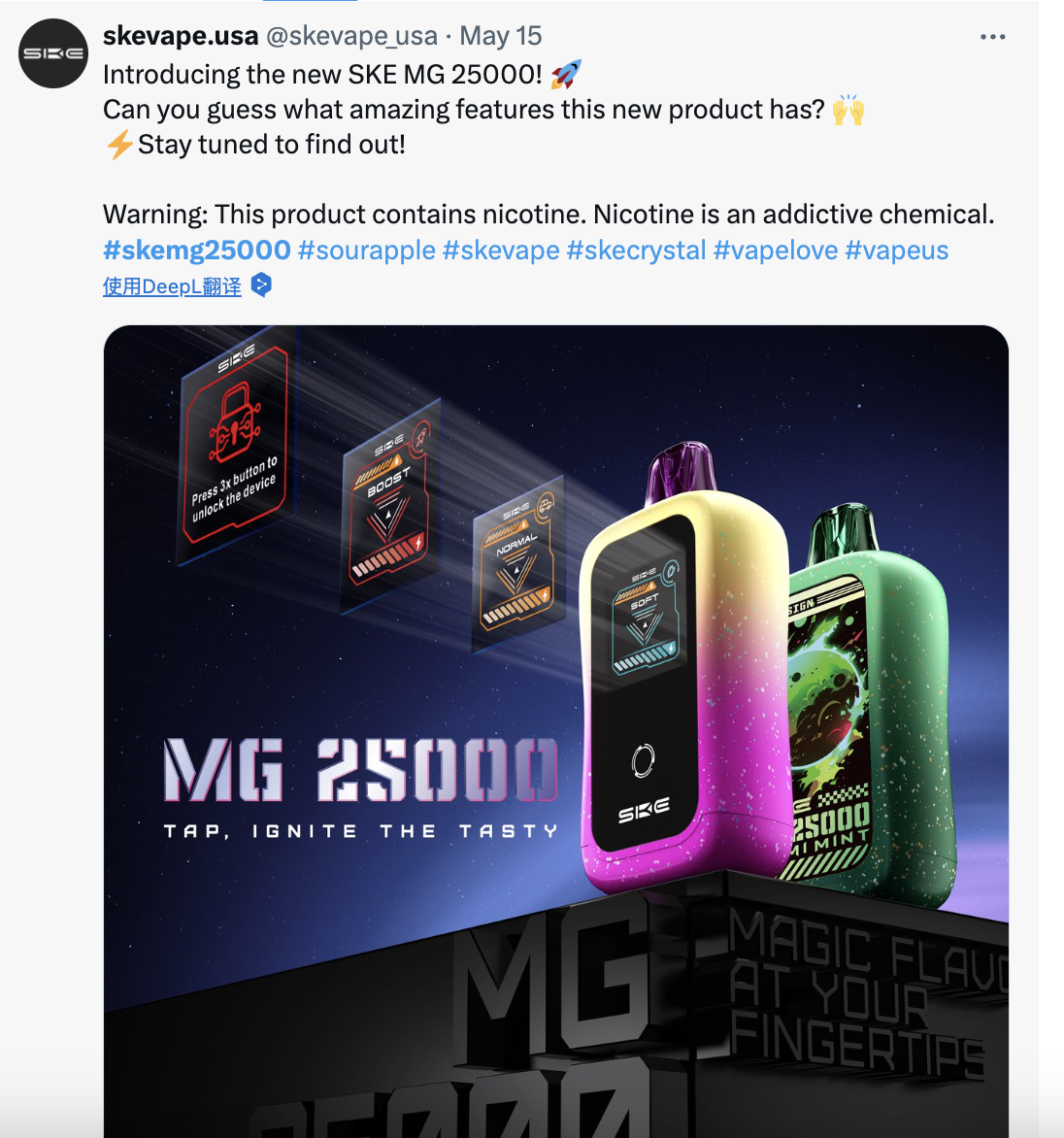
Another brand of Scour, VFLY, also launched its debut product, the VFLY C1, in May. According to the official website, the VFLY C1 is a disposable e-cigarette that claims to offer the "world's first real-time vaporization visual experience." The device's screen displays real-time battery usage, e-liquid remaining, and different modes. The design is similar to the circular interface of the Apple Watch. At that time, a representative from Scour introduced the market strategy of the SKE brand in the United States to 2Firsts.

The product is currently priced at $16.99 and features three adjustable modes: "Delicious (10W)," "Rich (14W)," and "Ultimate (18W)," with a maximum of 25,000 uses.
MEMERS made its debut at the TPE Expo in the United States, held from January 31 to February 2, 2024, where it showcased two products: Switcher S20000 and Melody ME18000. The SWITCHER S22000 is a disposable product with two e-liquid pods of 12ml each, totaling 24ml of e-liquid, offering approximately 22,000 puffs, and is currently priced at $13.89.
Below is the product information for the representative products of the three brands on vape sourcing:
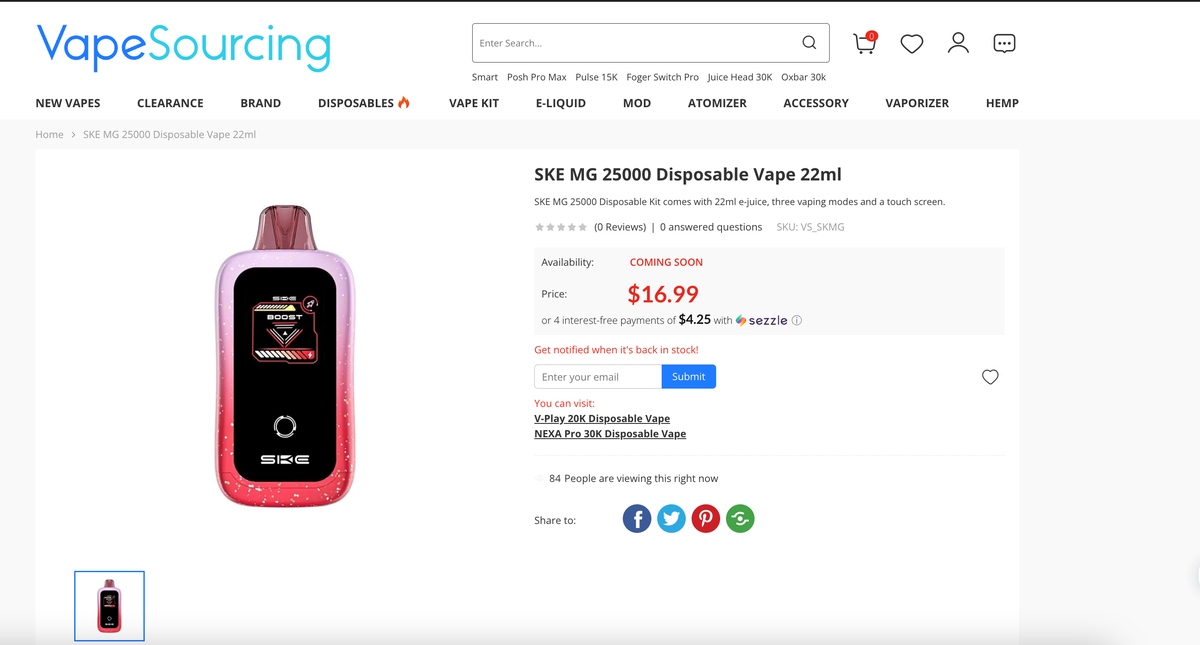
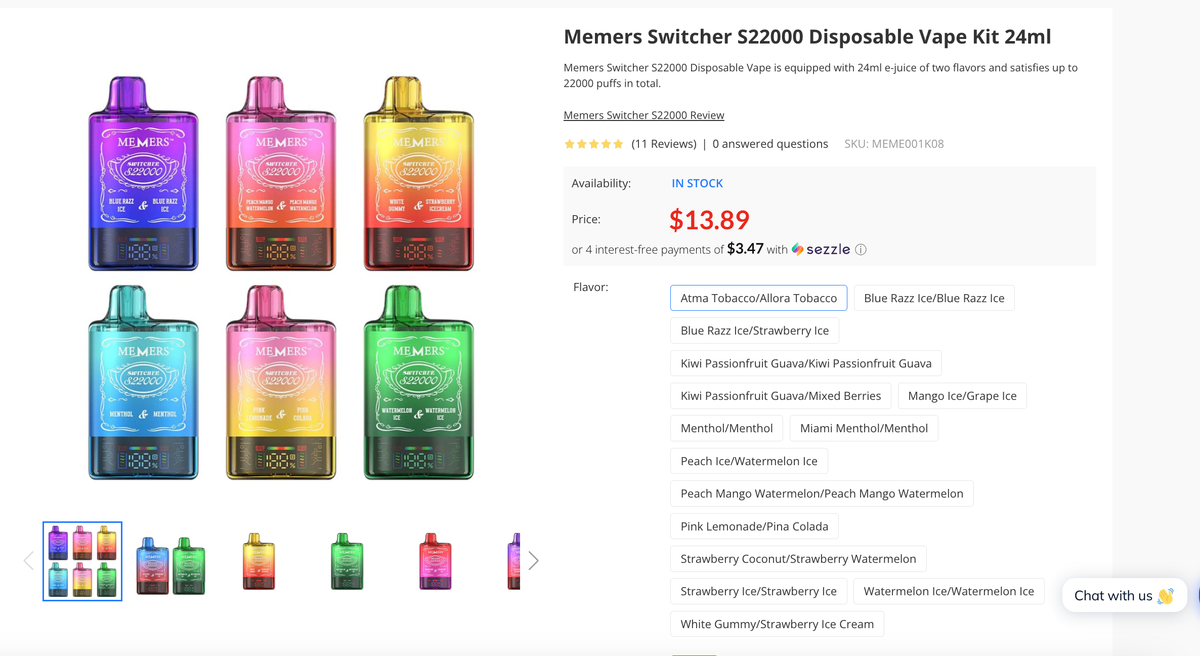
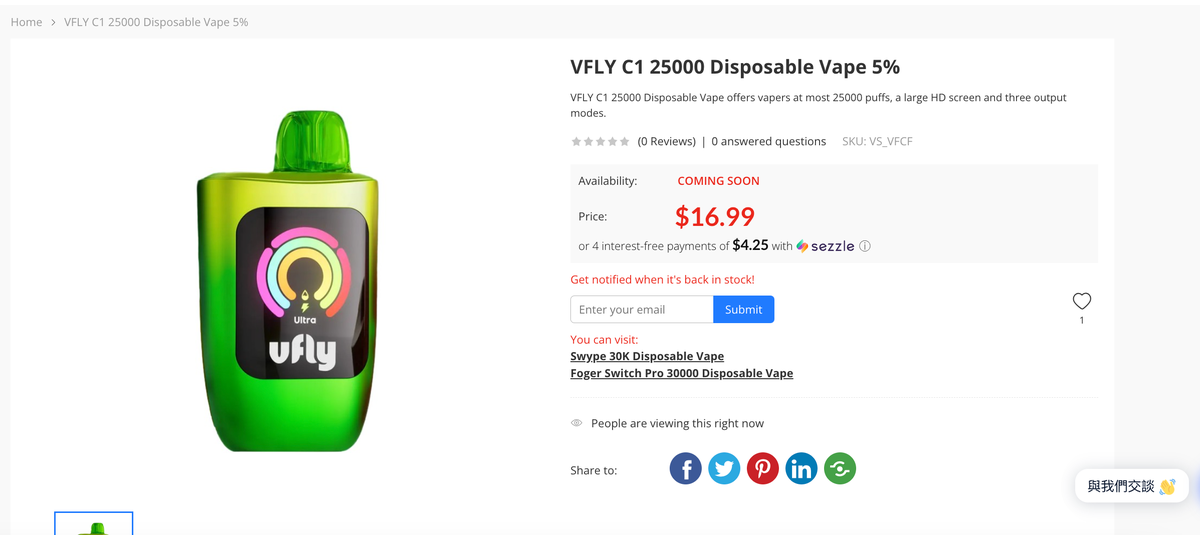
2Firsts also inquired with relevant personnel at Shenzhen Scour Technology Co., Ltd. about the details, who stated, "We have indeed 'passed the first phase of PMTA review and obtained STN authorization for two products.'"
However, they did not specify which brand's products received the STN numbers.
Whether Scour can ultimately gain a share of the American market remains to be seen. 2Firsts will continue to monitor Scour's market activities in the United States.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








