
Special statement:
This article is for internal industry communication only and does not make any recommendations for specific brands or products.
The images presented in this article are only used to describe the facts and are not intended as advertisements for any products.
Minors are prohibited from accessing this article.
Key Highlights
- Approvals Decline Week-on-Week: A total of 317 e-cigarette registration codes were approved between September 15 and 21.
- Brands Intensify Efforts: New devices from popular brands like IVG and LOST MARY were prominently featured, while FREEMAX products were approved across multiple categories.
- Pod Approvals Plummet: The number of pod registration codes saw a significant drop of approximately 82% compared to the previous week. JUUL pods were approved for the first time since January 2025.
- Comprehensive Product Coverage: The approved products include rechargeable devices, refillable vape systems, complete kits, and standalone components.
2Firsts, September 22, 2025 -In the UK, e-cigarette products must be registered in the Medicines and Healthcare products Regulatory Agency (MHRA) database. Once an item is registered, it means the product has passed compliance checks and is legally permitted for sale in the UK market.
To help the industry understand the status of new products in the UK, 2Firsts regularly compiles and analyzes information from MHRA notifications.
Below is an analysis of the notices updated from September 15 to September 21, covering major brands, product types, and initial market trends.
During this period, the MHRA registered a total of 317 registration codes. Brands involved include IVG, LOST MARY, FREEMAX, CRYSTAL BAR, VAPES BARS, and more.
IVG and LOST MARY Launch New Devices
In the product category "Electronic cigarette – Rechargeable, placed on the market with one type of e-liquid (fixed combination). Any rechargeable which can also be used as a refillable should be reported under the refillable category," a total of 3 registration codes were updated from September 15 to September 21. These were for the brands BLACK DRAGON and ORTO.
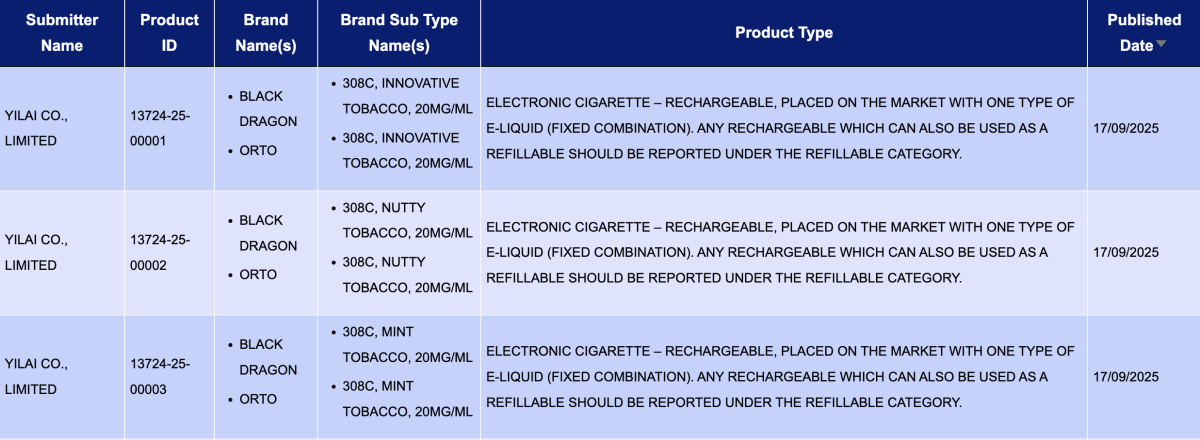
- YILAI CO., LIMITED, as the applicant, had 12 registration codes approved for its BLACK DRAGON and ORTO brands. The products come in three flavors—Innovative Tobacco, Nutty Tobacco, and Mint Tobacco—all with a nicotine strength of 20mg/ml.
In the product category "Electronic cigarette – Rechargeable, device only. Any rechargeable which can also be used as a refillable should be reported under the refillable category," a total of 4 registration codes were updated between September 15 and September 21. These were for brands like IVG and LOST MARY.
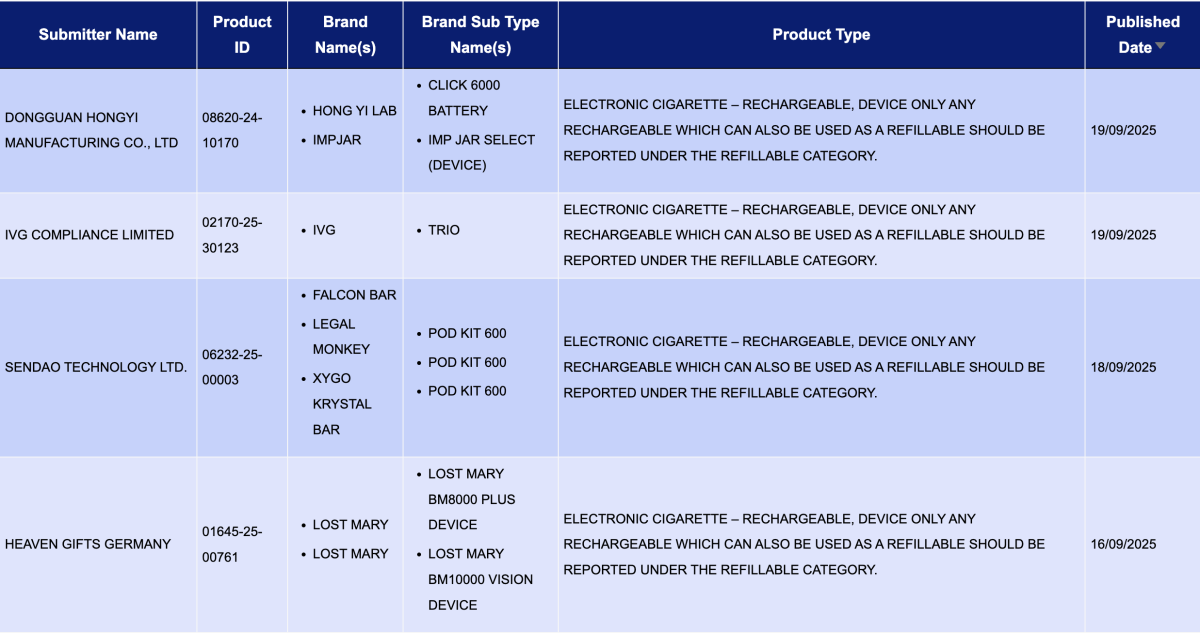
- IVG COMPLIANCE LIMITED, as the applicant, registered the IVG TRIO.
- HEAVEN GIFTS GERMANY, as the applicant, registered the LOST MARY BM8000 PLUS Device and the LOST MARY BM10000 VISION Device under the LOST MARY brand.
FREEMAX Products Approved in Multiple Categories
In the product category "Electronic cigarette – Refillable, device only," a total of 4 registration codes were updated between September 15 and September 21. These were from brands like FREEMAX and VAPES BARS.
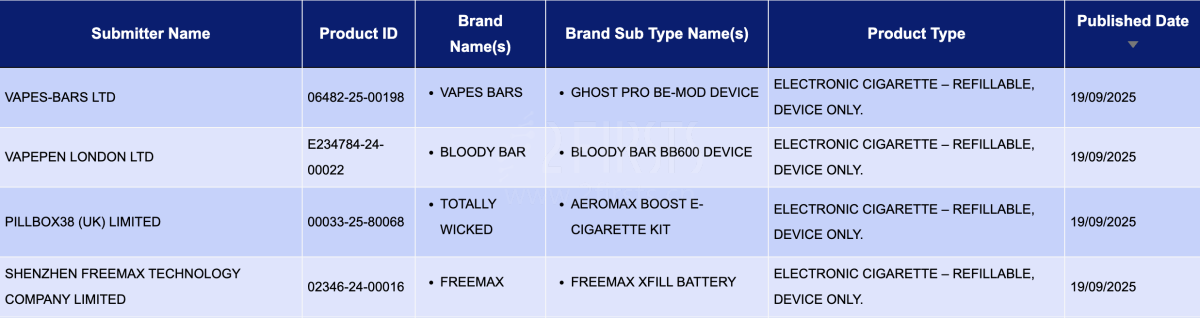
- VAPES-BARS LTD, as the applicant, registered the VAPES BARS Ghost Pro BE-MOD Device.
- VAPEPEN LONDON LTD, as the applicant, registered the BLOODY BAR BB600 DEVICE under the BLOODY BAR brand.
- PILLBOX38 (UK) LIMITED, as the applicant, registered the Totally Wicked Aeromax Boost E-Cigarette Kit.
- SHENZHEN FREEMAX TECHNOLOGY COMPANY LIMITED, as the applicant, registered the Freemax Xfill Battery under the FREEMAX brand.
In the product category "Kit – Pack containing more than one different e-cigarette device and/or more than one different refill container/cartridge," a total of 5 registration codes were updated from September 15 to September 21. These were from the FREEMAX and IVG brands.
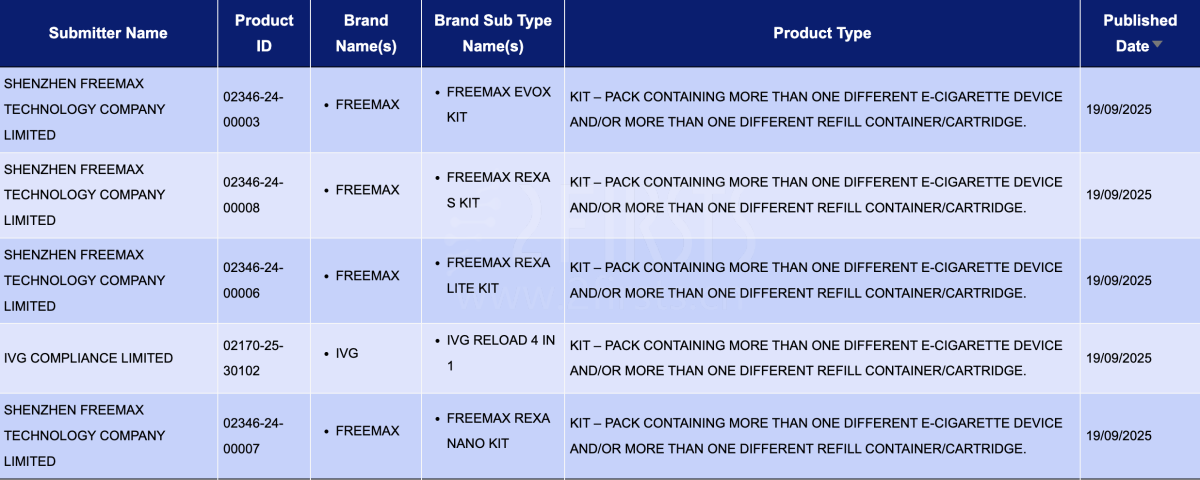
SHENZHEN FREEMAX TECHNOLOGY COMPANY LIMITED, as the applicant, had 4 registration codes updated for the following FREEMAX products:
- Freemax EVOX Kit
- Freemax REXA S Kit
- Freemax Rexa lite Kit
- Freemax REXA NANO Kit
IVG COMPLIANCE LIMITED, as the applicant, registered the IVG RELOAD 4 in 1 under the IVG brand.
In the product category "Individual part of electronic cigarette capable of containing e-liquid," a total of 46 registration codes were updated between September 15 and September 21. These were from brands like FREEMAX and CRYSTAL BAR.
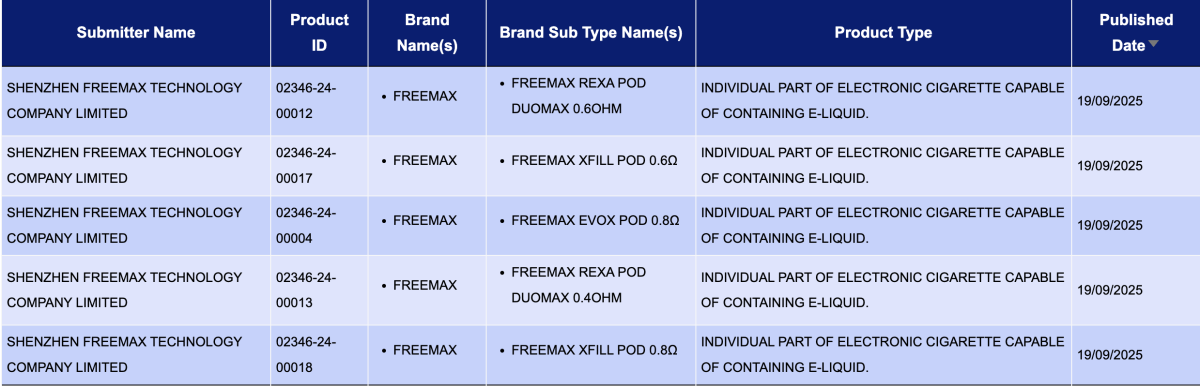
SHENZHEN FREEMAX TECHNOLOGY COMPANY LIMITED, as the applicant, had 8 registration codes updated for the following FREEMAX products:
- FREEMAX REXA Pod DUOMAX 0.6ohm
- Freemax Xfill Pod 0.6Ω
- Freemax EVOX Pod 0.8Ω
- FREEMAX REXA Pod DUOMAX 0.4ohm
- Freemax Xfill Pod 0.8Ω
- Freemax EVOX Pod 0.6Ω
- FREEMAX REXA Pod 0.8ohm
- Freemax Xfill Chubby Bottle
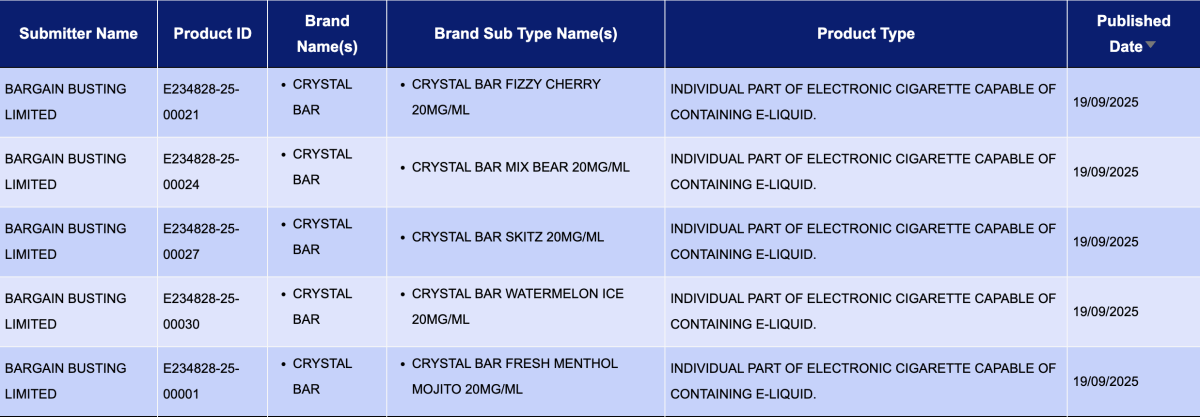
BARGAIN BUSTING LIMITED, as the applicant, had 32 registration codes updated for the CRYSTAL BAR brand, all with a nicotine strength of 20mg/mL. The products include:
- CRYSTAL BAR Fizzy Cherry
- CRYSTAL BAR MIX BEAR
- CRYSTAL BAR SKITZ
- CRYSTAL BAR Watermelon Ice
- CRYSTAL BAR Fresh menthol Mojito
- CRYSTAL BAR Strawberry Kiwi
- CRYSTAL BAR Banana Ice
- CRYSTAL BAR STRAWBERRY BURST
- CRYSTAL BAR Cola Ice
- CRYSTAL BAR Triple Mango
- …and 22 others.
Pod Approvals Down Approximately 82% JUUL Registers 5 Pod Codes
In the "Refill container/cartridge containing e-liquid" product category, a total of 255 registration codes were updated between September 15 and September 21. This included brands such as JUUL2, IVG, LOST MARY, and SUONON | CRYSTAL.
JUUL LABS UK HOLDCO LIMITED, as the applicant, registered 5 registration codes for the JUUL2 brand, all with a nicotine concentration of 18mg/mL.
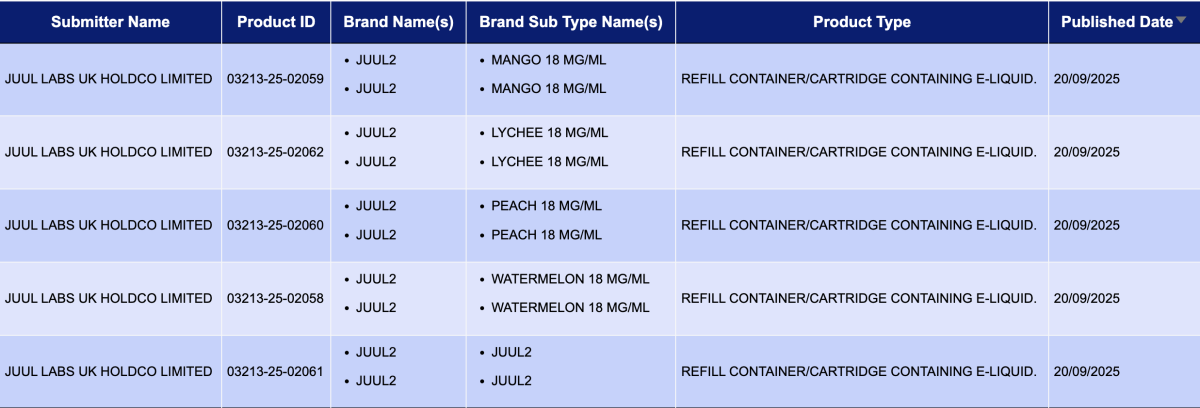
Compared to the 1,397 pods registered last week (September 8-14), the number of pod approvals this period has dropped significantly by approximately 82%.
As a leading global NGP media and think tank, 2Firsts is dedicated to providing the latest product and technological information and insights to practitioners around the world in various categories such as e-cigarettes, heated tobacco products, and modern oral products. It aims to drive technological changes and innovations in NGP products worldwide, thereby offering tobacco consumers globally with safer products and lifestyles.
With a source of information covering the supply chains in China and global markets, 2Firsts product coverage has become one of the most influential platforms for new product and technology releases globally.
Contact 2Firsts for the following services:
1. Providing leads on new products and technologies;
2. Offering comments on products and technologies;
3. Seeking media coverage for your products;
4. Identifying sales channels for products.
Contact Information: Email: info@2firsts.com
Contact CEO Alan Zhao of 2Firsts on LinkedIn.








