
As a media outlet dedicated to the long-term research and reporting of global NGP regulatory policies, industry economics, and market developments, 2Firsts provides the following Position:
1. This article focuses solely on the regulation and commercial issues of e-cigarettes and is intended for readers within the global tobacco industry.This article does not involve analysis or commentary on any political or diplomatic issues.
2. The content of this article may not be quoted for political or diplomatic purposes.
3. The statements and views of the individuals mentioned in this article are presented objectively as a means of conveying information and do not necessarily reflect the opinions of 2Firsts. 2Firsts also does not evaluate the opinions of these individuals in other areas in this article.
4. The Chinese translation of the U.S. Congressional materials cited in this article is for reference only. All content should be based on the original English text.
【2Firsts】On December 4th, U.S. Congressman Raja Krishnamoorthi announced the official launch of an investigation into the import of illegal e-cigarettes and sent out inquiry letters to five Chinese manufacturers and six American wholesalers and distributors.
The investigated Chinese manufacturers were required to provide a series of data and information, including global revenue for the past three years, sales data to the United States, shareholder information, taxes paid to the government and subsidies received, customs brokers, distributors, retailer information, product details, and research on safety, etc. The inquiry letter to the Chinese manufacturers was also copied to the Commissioner of the U.S. Food and Drug Administration (FDA), the Acting Commissioner of the U.S. Customs and Border Protection (CBP), and the Attorney General of the Department of Justice (DOJ).
The six U.S. wholesalers and distributors are required to provide a list of Chinese-made e-cigarettes they represent, revenue data, communication records with Chinese e-cigarette manufacturers regarding compliance over the past three years, and details of their compliance processes. Additionally, these U.S. wholesalers and distributors must explain why unauthorized products have been sold.
In the press release of the U.S. Congress, the above actions were explained as part of Congressman Raja Krishnamoorthi's "larger campaign to end all forms of youth vaping."
This is not Congressman Raja Krishnamoorthi's first action. At the beginning of December, Congressman Raja Krishnamoorthi publicly stated:
"The endless supply of fruity, bright e-cigarettes found in so many store fronts are illegal. That is shocking and unacceptable, and the FDA must immediately use their authority to pull all illegal products from the shelves. I know that youth vaping is a public health crisis, and the actions of companies addicting our kids with flavors and impossibly high levels of nicotine are endangering their health and their lives."
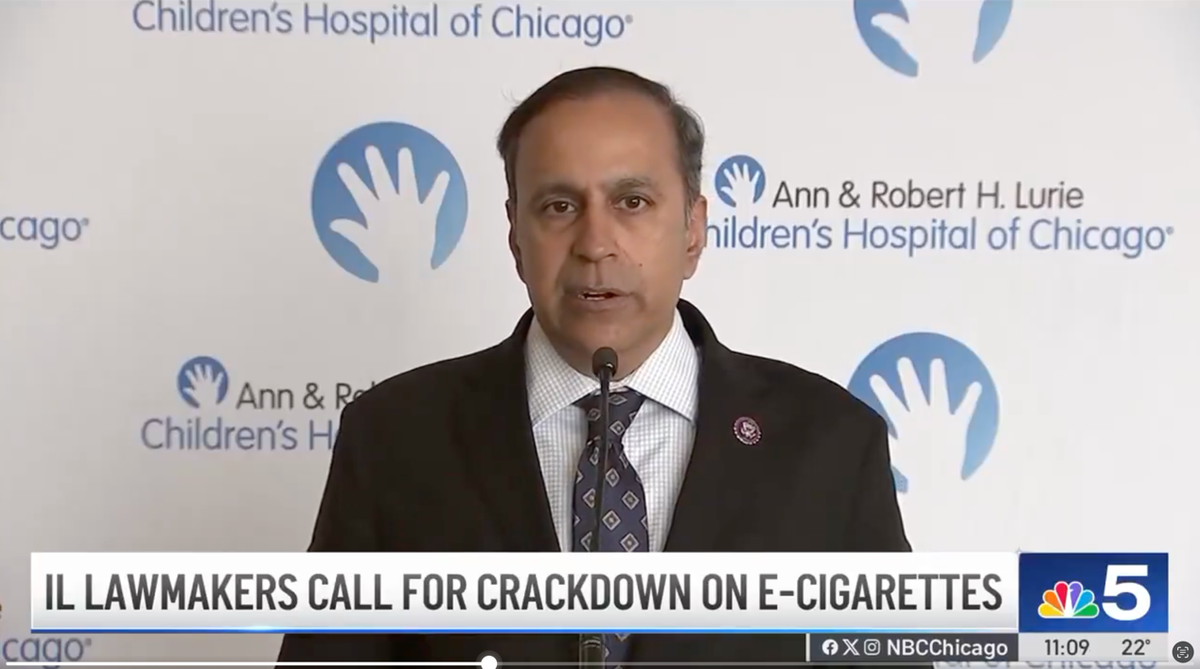
In an earlier instance, on April 10, 2024, Congressman Raja Krishnamoorthi and Congresswoman Celeste Maloy spearheaded a letter to the Department of Justice (DOJ), urging stronger enforcement and prosecution against illegal flavored e-cigarette products that lack authorization from the U.S. Food and Drug Administration (FDA). The letter also called for an expansion of the scale of e-cigarette enforcement activities.
Additionally, the DOJ was requested to provide various enforcement details, such as the number of prosecutions related to e-cigarettes, the methods of collaboration with the FDA, and whether a dedicated task force exists to combat illegal e-cigarette crimes.
Moreover, in the 2019 investigation of JUUL's illegal marketing to minors, Raja Krishnamoorthi played a key role.
Upon inquiry, Guangdong Qisitech Co., Ltd. issued a statement on an international e-cigarette media outlet on December 10th.
Five Chinese Manufacturers Investigated
In this inquiry, Congressman Raja Krishnamoorthi proposed sending letters to five Chinese manufacturers and specifically named the brands.
1). Guangdong Qisitech Co. Ltd (Geek Bar and Raz)
2). Shenzhen HanQingDa Technology Co. Ltd (HQD Cuvie)
3). Shenzhen Heaven Gifts Technology Co., Ltd (Elf Bar, EB Design, Lost Mary)
4). BFL Metal Production Ltd (Fume)
5). Dongguan Delin Technology Co. Ltd (Juicy Bar)
The letter posed specific questions to five Chinese manufacturers, requesting that these companies respond and submit relevant documents by January 1, 2025.
(The bolded sections before each question were added by 2Firsts for ease of reading. Please refer to the original text for accuracy.)
1. Income details: Please describe your global revenue in each of 2022, 2023, and 2024 to date. Please further identify what percentage of your global revenue is directly or indirectly traceable to exports to the United States.
2. Shareholder information: Please identify each shareholder in your company (or any holding company, parent company, or subsidiary) with a 1% or greater ownership interest.
3. Tax Payment: Please provide specific dollar or yuan amounts paid in 2022, 2023, and 2024 to date with respect to each of the following entities:
a. The United States Government and any intermediary acting on its behalf (including for taxes, import fees, duties, and other required payments); and
b. The Government of the People’s Republic of China (including any provincial government) and any intermediary acting on its behalf (including for taxes or other amounts payable to the China National Tobacco Company).
4. Government support: Please describe any subsidies, funding, or other benefits your company receives from any government entity in the People’s Republic of China.
5. Legal Compliance: Please describe all efforts your company has made to comply with U.S. law and regulations related to ENDS products.
6. Transportation and labeling: Please describe how your company labels products intended for shipment to the United States and provide a list of all goods and harmonized tariff codes your company uses on import labels, including corresponding invoice identification numbers.
7. Distribution network: Please provide a list of all customs brokers and distributors used to import and sell your products in the United States.
8. Retailer list: Please provide a list of all retailers in the United States that you supply.
9. Safety research: Please describe any research you or a third party have conducted regarding the safety of your products, including the impact of such products on children under the age of 18.
10. Product list: Please provide a full list of all brands and products produced by your company. Please indicate which flavors (including, but not limited to, flavors known to be attractive to children under the age of 18) and nicotine levels you produce for each brand and product.

Six American Vape Distributors and Wholesalers Investigated
At the same time, six American e-cigarette wholesalers and distributors also received similar letters, including:
1. Midwest Goods
2. Kanger Wholesale
3. Vape Wholesale USA
4. Demand Vape
5. World Vape USA
6. Mi-Pod
Members of Parliament have requested detailed information from these companies in the letter, including their association with illegal products, the legality of sales channels, and whether they have marketing behaviors targeting minors. And require a response by the same deadline:
1. Distribution compliance: Does your company currently distribute or otherwise sell tobacco products, including ENDS products, from the PRC that are not authorized by FDA? If your company denies that it currently sells such unauthorized products, please provide an explanation for the appearance of such products on your website.
2. Review process: Please describe your company’s process for ensuring that the products that you sell have received FDA authorization.
3. Communication records: Please provide copies of any correspondence sent or received from January 1, 2022 to present related to ENDS products between your company and any PRC-based entity regarding the compliance or noncompliance of such products with U.S. laws or regulation. If no such correspondence exists, please explicitly state this.
4. Removal of illegal products: Please describe your company’s process for removing non-FDA-authorized products from distribution, including such products made in the PRC or produced by PRC-based entities.
5. Product list: Please provide a full list of all PRC-based brands and products distributed by your company. Please indicate which flavors (including, but not limited to, flavors known to be attractive to children under the age of 18) and nicotine levels you distribute for each PRC-based brand and product.
6. Shipping description: Please describe how shipments of ENDS products your company receives from the PRC and labeled and/or manifested.
7. Revenue details: Please describe your revenue in each of 2022, 2023, and 2024 to date. Please further identify the amount of revenue your company obtained by distributing PRC-produced ENDS products that have not been authorized by the FDA for each such year.

Independent Commentary by 2Firsts:
In the statements of Congressman Raja Krishnamoorthi and the inquiry letters sent to Chinese e-cigarette manufacturers, the following expressions appear multiple times:
"An overwhelming percentage of the illegal e-cigarettes in the U.S. are made in the PRC."
"In 2022, the PRC banned flavored e-cigarettes from being sold within China. However, the PRC did not similarly prohibit the export of these dangerous products to the U.S. or other jurisdictions."
As an institution with extensive experience studying China’s e-cigarette regulatory policies, 2Firsts provides the following position:
1. China is the center of the global e-cigarette supply chain, a position established as a result of global industrial division of labor, in accordance with market economy principles and international trade rules.
2. The illegal actions of certain Chinese manufacturers should not be generalized to represent all “Made in China” products. In fact, the vast majority of compliant e-cigarette brands, including those of international tobacco companies, are also manufactured in China.
3. China’s e-cigarette regulatory policies prohibit the sale of flavored e-cigarettes within its domestic market. For international markets, considering the diversity of regulatory policies and product standards across countries (in fact, flavored e-cigarettes remain legal in many jurisdictions), China’s regulatory authorities cannot mandate foreign markets to adopt the same policies and standards as those enforced domestically. However, Chinese e-cigarette regulatory laws explicitly require enterprises to comply with the laws of their destination countries.
4. The issue of illegal trade in e-cigarettes is a global challenge. As illegal trade often involves complex chains and actors, effectively regulating such activities requires international cooperation among regulators. As Alan Zhao, CEO of 2Firsts, emphasized in his 2024 speech at the Global Forum on Nicotine (GFN), regulatory authorities worldwide should strengthen collaboration and establish a global regulatory coordination mechanism to address illegal e-cigarette trade.
The above statement reflects the views of 2Firsts alone and does not represent any official position.
Feel free to provide clues and comments, please contact: info@2firsts.com, or contact 2Firsts CEO Alan Zhao on LinkedIn.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








