
The Vapor Technology Association (VTA) recently praised President-elect Donald Trump's nomination of Robert F. Kennedy Jr. as Secretary of Health and Human Services (HHS), calling it a demonstration of decisive leadership.
VTA stated that the nomination reflects Trump's commitment to addressing government inefficiencies, particularly the bureaucratic regulatory processes led by FDA and the Center for Tobacco Products (CTP).

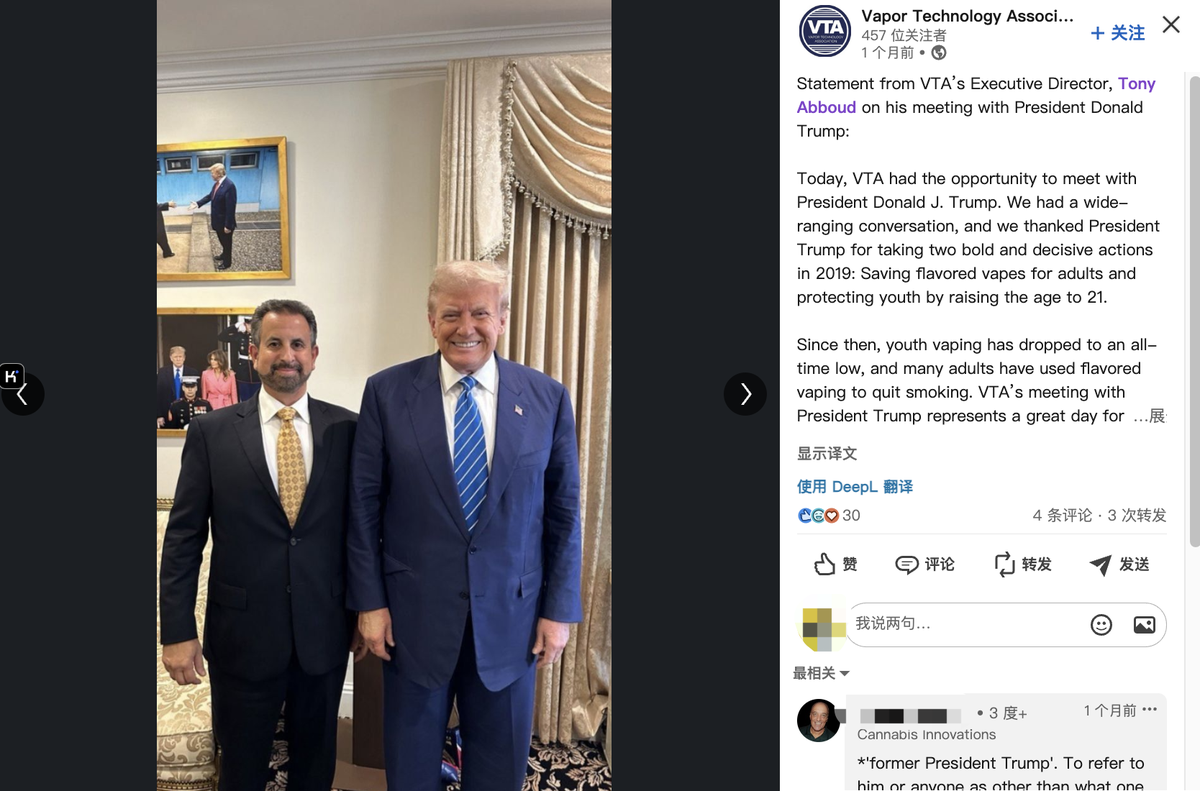
"President Trump has vowed to save flavored vaping and RFK has vowed to Make America Healthy Again by ending chronic diseases driven by the industrial complex. The thousands of small businesses in the vapor industry, the hundreds of thousands of Americans they employ, and the tens of millions of Americans who depend on the industry for freedom to choose less harmful flavored nicotine alternatives to quit smoking, are counting on President Trump, RFK, and the rest of the Trump administration, VTA Executive Director Tony Abboud said.
The VTA is calling on the government "to implement a new, streamlined regulatory process that elevates science over politics, that ensures broad access to flavored vapes to end chronic smoking related diseases, and that eliminates bureaucratic conflicts of interest with transparent and rational scientific standards to fix the FDA's current broken regulatory framework. Swift and decisive action can save 500,000 American lives lost to cigarettes every year."

Previously, VTA claimed that its e-cigarette voters helped conservative party candidates secure victory in the election. During this election, Abd met face-to-face with Donald Trump, and VTA released a statement at the time thanking Trump for taking two bold and decisive actions in 2019: saving flavored e-cigarettes for adult use and raising the age to 21 to protect teenagers.
After the meeting, Trump stated on the social media platform Truth Social:
"I saved Flavored Vaping in 2019, and it greatly!helped people get off smoking.l raised the age to21, keeping it away from the "kids." Kamala and Joe want everything banned, killing small businesses allover the Country. i'll save Vaping again!"
According to its official website, the Vapor Technology Association (VTA) is a non-profit trade group founded in Washington, D.C., in 2015. It focuses on advancing the vapor product industry, advocating for science-based regulatory policies, and ensuring adult consumers' access to safer nicotine alternatives. Its membership includes device manufacturers, e-liquid producers, distributors, suppliers, and retailers.








