
The 2024 US presidential election has concluded, with Donald Trump securing over 270 electoral votes to win a second term. On November 7, Vapor Technology Association (VTA) posted an announcement on LinkedIn titled "VTA' SIVAPEIVOTE CAMPAIGN DELIVERS SUCCESS FOR CONSERVATIVE CANDIDATES" claiming that vaping voters helped conservative candidates win the election.

Here is the original announcement released by the VTA.

VTA' SIVAPEIVOTE CAMPAIGN DELIVERS SUCCESS FOR CONSERVATIVE CANDIDATES
- "Vaper voters showed up in droves to support Conservative candidates who will protect and preserve therights of Americans to use flavored vaping products."
- "VTA's l Vape l Vote campaign made clear that vaper voters had their voices heard at the ballot box and ensured that Conservative candidates would deliver full-throated endorsements of Americans' right to use flavored vapes and, critically, use that support to establish a voter currency which propelled several Conservative candidates into office."
- "While we are proud to have engaged in the process with several of these Conservative candidates, we are now ready to see their campaign promises committed to action as they work with President-elect Trump and the relevantfederal agencies to fix the broken regulatory process by implementing a streamlined regulatory processthat ensures access to flavored vapes is protected and companies and distributors have transparent,rational, and affordable rules of the road when it comes to this regulatory framework."
-Executive Director Tony Abboud
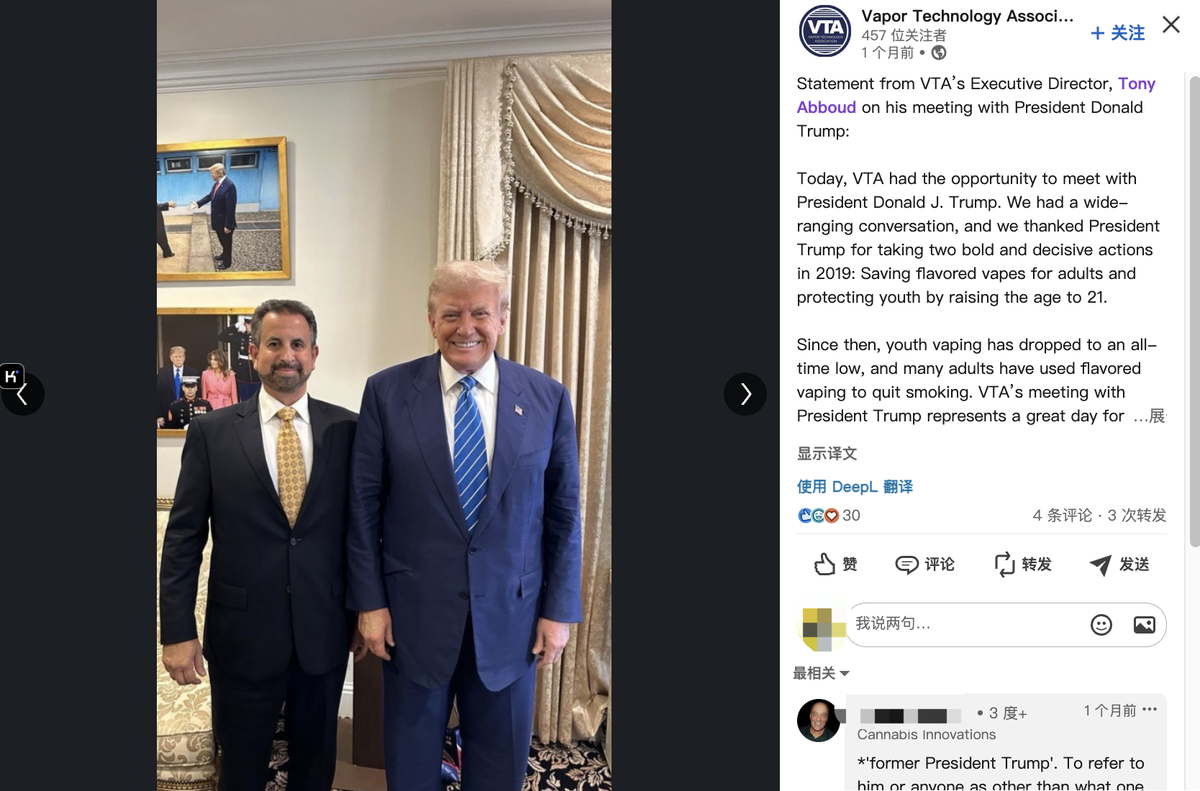
2Firsts noted that during the election, VTA Executive Director Tony Abboud met with Donald Trump in person. At the time, VTA issued a statement stating that "Today, VTA had the opportunity to meet with President Donald J. Trump. We had a wide-ranging conversation, and we thanked President Trump for taking two bold and decisive actions in 2019: Saving flavored vapes for adults and protecting youth by raising the age to 21."
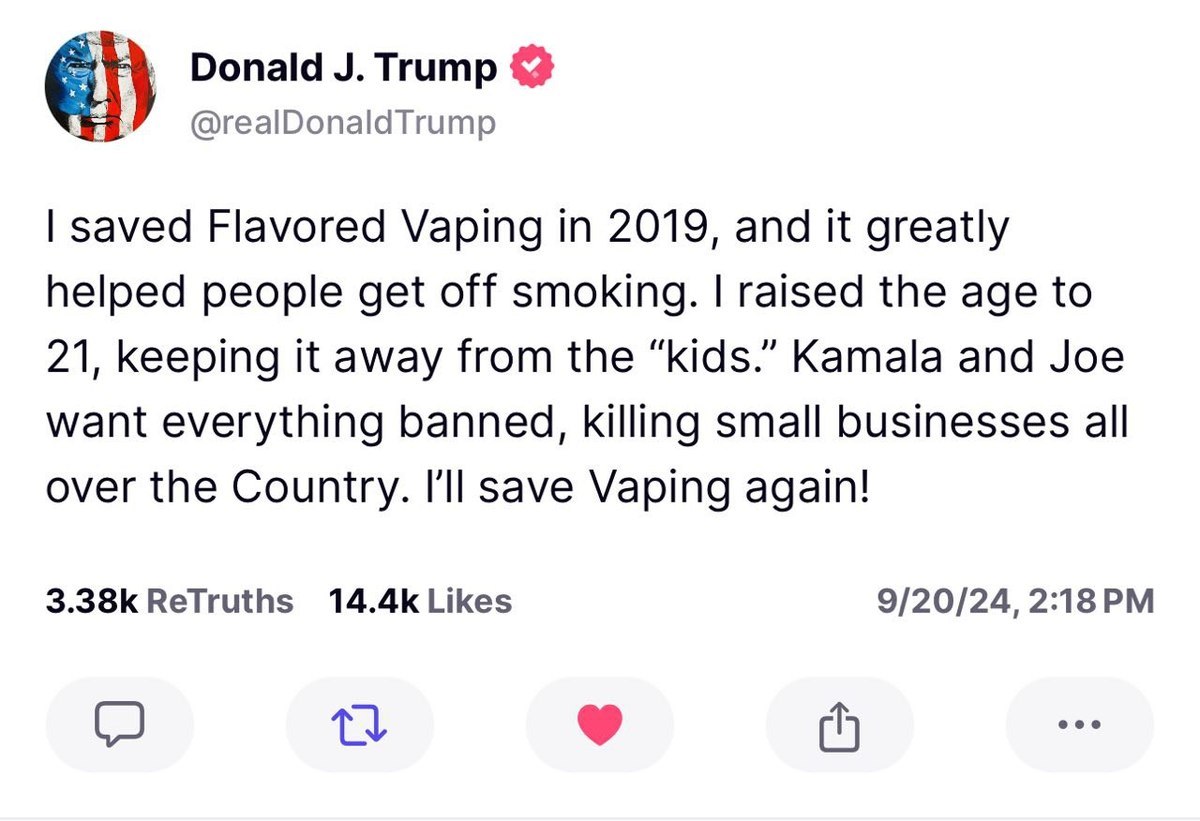
After the meeting, Trump stated on the social media platform Truth Social:
"I saved Flavored Vaping in 2019, and it greatly!helped people get off smoking.l raised the age to21, keeping it away from the "kids." Kamala and Joe want everything banned, killing small businesses allover the Country. i'll save Vaping again!"
"Since then, youth vaping has dropped to an all-time low, and many adults have used flavored vaping to quit smoking. VTA’s meeting with President Trump represents a great day for small businesses across America who fear the Biden-Harris administration’s efforts to shut down small businesses and deprive adults who smoke of their flavored vaping products. We are pleased that former President Trump is continuing to fight for vapers," VTA said on its social media.
On VTA website, as well as on social media platforms like X and Facebook, it posted multiple graphic and text posts calling on its followers to participate in the "I VAPE I VOTE" campaign.

According to its official website, VTA is a non-profit trade association founded in 2015 in Washington, D.C. It is dedicated to supporting the development of the vaping products industry, advocating for scientifically sound regulations, and ensuring that adult consumers have access to safer nicotine alternatives. Its members include device manufacturers, e-liquid producers, distributors, suppliers, and retailers.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








