
The 2025 NGP Global Compliance Development New Year Symposium, organized by 2Firsts, successfully concluded in the Qianhai area of Shenzhen on January 9, 2025. The theme of the symposium was "Compliance Drives Growth," focusing on the cutting-edge trends in global NGP compliance development, with the goal of promoting the compliance development of the global NGP industry in 2025.

The symposium invited more than 10 industry experts and compliance representatives from around the world to deliver keynote speeches in a hybrid format of online and offline. It attracted nearly 200 representatives from international tobacco companies, leading Chinese e-cigarette companies, and other businesses to participate.
Global Compliance Experts Analyze Global Compliance Trends
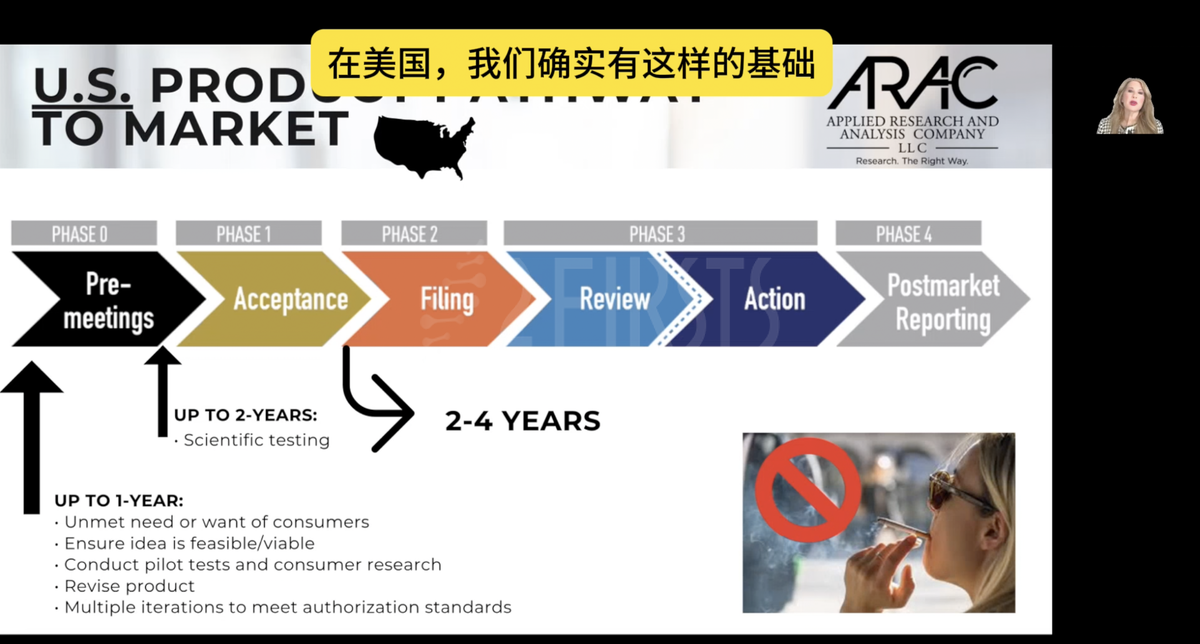
Azim Chowdhury, a partner at the law firm Keller & Heckman, delivered a speech on the topic of "PMTA Opportunities and Challenges in 2025." With his rich industry experience and professional insights, he comprehensively analyzed the compliance situation of the US e-cigarette market and made scientific predictions about future compliance trends.
He stated that the product standards set by the PMTA for flavored e-cigarettes are extremely strict, and stakeholders must demonstrate their benefit in helping adult smokers quit. At the same time, he also emphasized the importance of scientific evidence and device access restrictions in obtaining FDA authorization, and urged companies to actively participate in the PMTA process to ensure compliance and avoid enforcement actions.

Dr. Jessica Zdinak, Chief Research Officer and CEO of ARAC, gave a presentation on the "Challenges and Considerations for Companies Applying for PMTA in 2025," detailing key aspects of the PMTA application process. She analyzed the opportunities and challenges facing businesses, and provided targeted strategies to help improve the success rate of PMTA applications. She predicted that the FDA is expected to review PMTA applications more efficiently and crack down on unauthorized overseas products entering the market.
The Secretary General of the Indonesia Personal Vaporizer Association (APVI), Garindra Kartasasmita, delivered a speech titled "2025 Trends in Indonesia's E-Cigarette Compliance" in which he analyzed the latest regulatory developments and future trends in the Indonesian e-cigarette market. His insights provide companies with precise guidance on compliance strategies in the country's market.

Dr. Andrea Patton, Ph.D. Behavioural Epidemiologist and Head of Prevalence Research at CSUR, a renowned research institution, gave a lecture on the topic "Opportunities and Challenges for Companies in Applying for PMTA in 2025." She approached the subject from an epidemiological perspective, delving deep into the impact of PMTA applications on product safety evaluations for businesses. Dr. Patton shared valuable insights and suggestions, such as the importance of meeting the APPH standards when applying for PMTAs in 2025. These recommendations are aimed at helping businesses effectively address challenges and seize opportunities during the application process.

Dr. Charlene Liu, founder of RiskWise Solution, presented on the topic of "The Implications of FDA's 2024 Scientific Policy Memorandum on Compliance Development in the e-cigarette Industry." During the presentation, she detailed the regulatory requirements set by the FDA for e-cigarette products, particularly focusing on how to ensure products meet the standards of protecting public health through quantitative risk assessments.

In addition, Dr. Charlene Liu also interacted with the live audience in the United States through a video link. The interactive session on this topic was hosted by Dr. Xinan Liu, a researcher at the Brain Institute of the Advanced Institute of Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences. With guidance from the two scientists, the audience participated in a deep discussion.

Top Chinese Regulatory Agencies Share Guidelines on Compliant Outbound Investments
At this symposium, several leading e-cigarette testing agencies in China participated, focusing on major global markets such as Europe, Southeast Asia, the Philippines, and Australia. They discussed key elements such as regulatory frameworks for NGP products, compliance standards, and market access conditions. They delivered a series of highly insightful and forward-looking keynote speeches, providing vital guidance for the industry's development.
Dr. Liu Yujun, the Technical Director of Tianjian Testing and a Ph.D. from The Chinese University of Hong Kong, delivered a speech titled "New Regulatory Trends of European NGP in 2025." With her extensive expertise and precise insight into the European market, she provided in-depth analysis of the new trends in compliance in the European novel tobacco market for 2025. She not only shared clear guidance on the regulatory approval for innovative products like 2+10 in Europe, but also made predictions on the future regulatory trends for NGPs such as nicotine pouches in Europe.

Chen Xiaoyan, manager of the Element Testing Research and Development Department, delivered a speech titled "Insights and Recommendations on Compliance Trends in the Southeast Asia Market 2025". Based on her in-depth research and rich development experience in the Southeast Asia market, she elaborated on the e-cigarette access regulations in Malaysia and Indonesia.
She stated that although the Southeast Asian market faces stringent regulation, the e-cigarette market in the region continues to show growth. In Malaysia, the percentage of e-cigarette users has significantly increased, and the market is expected to continue expanding. However, the refinement of regulations and the raising of market entry barriers have also brought new challenges to the industry's development.

Webb Testing General Manager Xiong Minghui delivered a keynote speech titled "Insights and Recommendations on Compliance Trends in the Philippines Market 2025." He pointed out that regulations in the Philippines e-cigarette market have been constantly updated in recent years, impacting manufacturers in various ways such as ICC logos no longer being recognized, product capacity needing to be specified, and certification marks needing to be updated.
Subsequently, he conducted a detailed analysis on the testing period, packaging design guidelines, product risk assessments, and factory capacity assessments related to the Philippine market, providing clear guidance for industry professionals operating in the Philippine market.

Frank Lin, the Technical Director of IMPAQ Testing's e-cigarette project, delivered a keynote speech titled "Insights and Recommendations on Compliance Trends in the Australian Market by 2025." Drawing on his rich experience and technical expertise in the e-cigarette industry, he comprehensively interpreted the compliance trends in the Australian market for 2025. He meticulously traced the timeline of the introduction of regulations for e-cigarettes in Australia and provided detailed explanations of regulatory requirements in various aspects such as ingredients, packaging, and containers in this market.

Interpretation of the regulation of e-cigarette operating permits in China and the problems of industry optimization and restructuring
Tang Shunliang, lawyer at Tianyuan Law Firm and expert in compliance in the NGP industry, delivered a keynote speech titled "Regulation of e-cigarette operating permits in China and compliance issues in industry optimization and restructuring". He provided a detailed explanation of the seven major risks of changing e-cigarette licenses for attendees, and emphasized the risks of companies violating regulations by combining practical cases, urging companies to operate in compliance.


2FIRSTS Releases the Top 10 Global Tobacco News for 2024

As a leading global NGP media and consultancy, 2Firsts announced the top ten news events in the global tobacco industry for 2024 at this symposium. They reviewed the ten key events that had a profound impact on the industry over the past year, helping industry professionals understand trends and prepare for future challenges.
2Firsts Annual Review: Top 10 Global NGP Industry Headlines for 2024
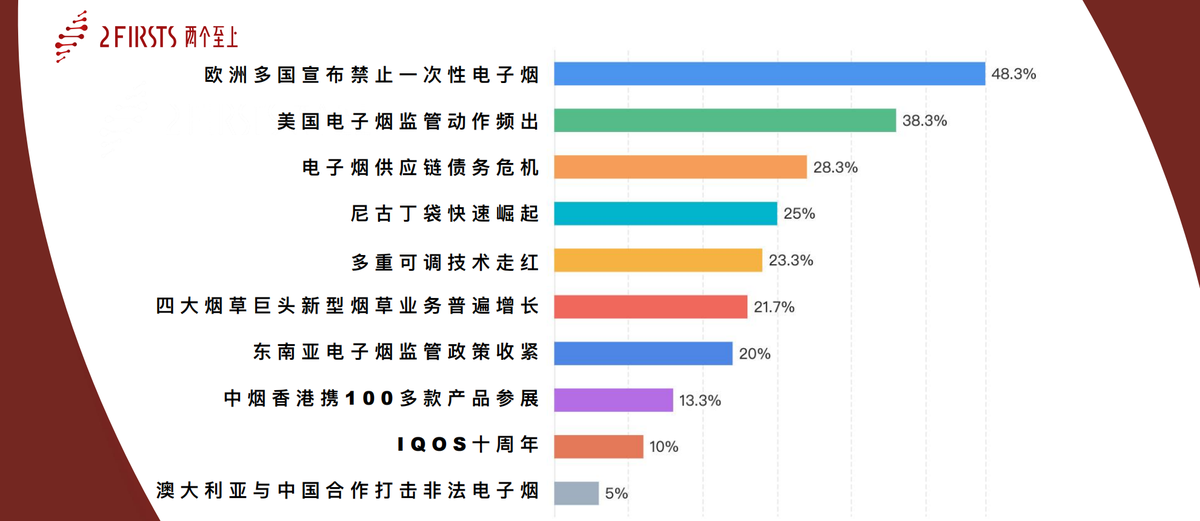
1. U.S. E-Cigarette Regulations Intensify, Setting the Stage for a Major Market Shakeup
2. Multiple European Nations Ban Disposable E-Cigarettes: A Potential Turning Point for High-Margin Products
3. Australia and China Collaborate to Combat Illicit E-Cigarette Trade: A Groundbreaking Global Enforcement Model
4. Southeast Asia Tightens E-Cigarette Regulations: Higher Barriers to Entry in Emerging Markets
5. Four Tobacco Giants Report Robust New-Product Growth: ‘Smoke-Free’ Strategies Take Center Stage
6. E-Cigarette Supply Chain Debt Crisis: Industry Shakeup Accelerates
7. CTIHK Showcases Over 100 Products: ‘Chinese-Style Heated Cigarettes’ Go Global
8. IQOS Turns Ten: Global Heat-Not-Burn Market Nears Critical Mass
9. Nicotine Pouches Surge in Popularity, Heightening Regulatory Uncertainty
10. Multi-Adjust Technology Gains Traction: E-Cigarette Consumption Enters a Customized Era

At the announcement of the Top 10 Global NGP News, 2Firsts invited distinguished experts from various fields to provide in-depth analysis and commentary.
Former Tobacco Reporter editor-in-chief and current 2Firsts global editor-in-chief, Taco Tuinstra, shared his insights based on nearly 30 years of experience in the tobacco industry. Addressing the news item, “IQOS at 10: The Global Heated Tobacco Market Established and Poised for Growth,” he noted that PMI has set its sights on the U.S. market. With its latest version, IQOS Iluma, already submitted for FDA approval and expected to receive authorization in the second half of 2025, the product is likely to generate significant revenue for PMI while providing U.S. nicotine users with a reduced-harm alternative.

Meanwhile, Li Chen, a new consumption analyst from China Cinda Securities Research Institute, also shared his perspective. He highlighted two major trends in the 2024 global NGP market: tightening compliance globally and accelerating product iterations. Looking ahead to 2025, he predicted that compliant companies' stock prices would continue to see positive momentum, while companies with exceptional products would achieve higher-than-expected growth, ensuring a dynamic and thriving industry.

In addition to expert commentary, 2Firsts hosted a live voting session, inviting attendees to select what they considered the most important NGP news of 2024. The results were revealed on-site:
- “Several European Countries Ban Disposable E-Cigarettes: A Turning Point for Product Opportunities”
- “U.S. E-Cigarette Regulation Escalates: A Market on the Brink of Transformation”
- “E-Cigarette Supply Chain Debt Crisis: Accelerating Industry Restructuring”
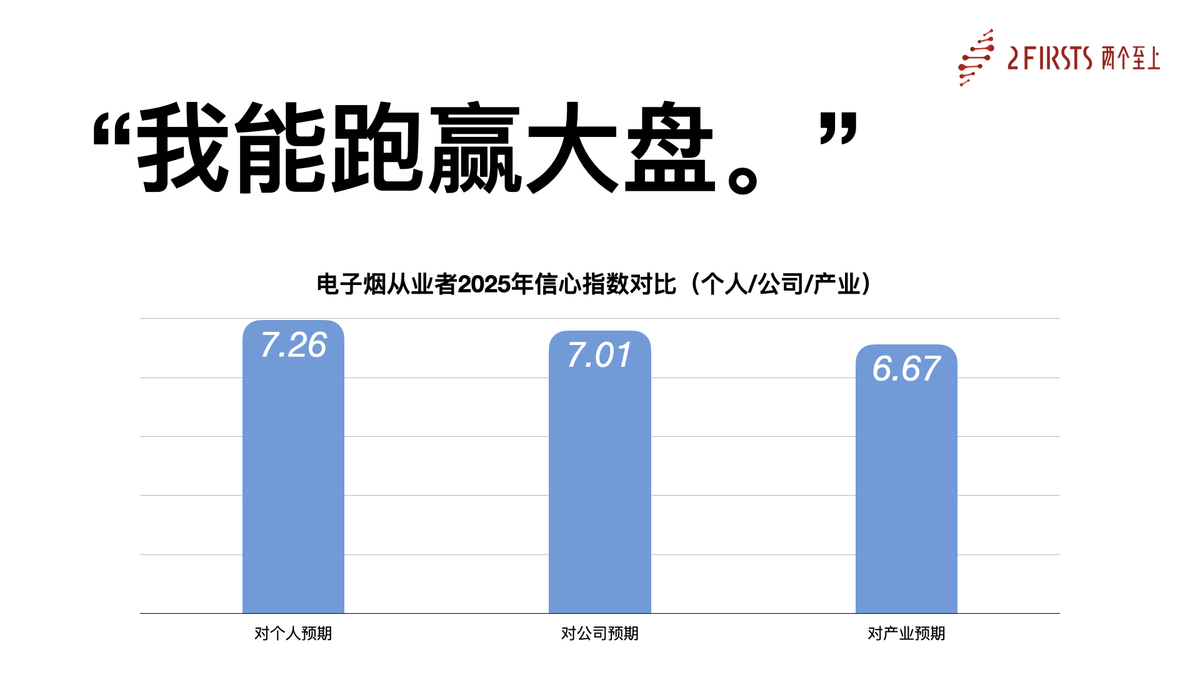
2Firsts Reveals Findings of the “2025 Confidence Index for China’s E-Cigarette Supply Chain Practitioners”
As the global supply chain hub for the e-cigarette industry, China's role in shaping the future of global NGP development is irreplaceable. In response to the evolving opportunities and challenges brought by global consumer demand shifts, 2Firsts launched the “2025 Confidence Index for China’s E-Cigarette Supply Chain Practitioners” survey at the end of 2024.
The survey aimed to assess the expectations and confidence levels of industry professionals regarding their individual, corporate, and industry-wide development in 2025.
At the seminar, 2Firsts unveiled the results of this research:
- Industry Confidence Index: 6.67
- Corporate Confidence Index: 7.01
- Personal Confidence Index: 7.26
2Firsts and Heno Bio Partner for Compliance Strategy
At the event, leading nicotine company Heno Bio and 2Firsts held a compliance strategy partnership launch ceremony, aiming to drive industry compliance and create a brighter future for the global e-cigarette industry.
Liu Qizhou, General Manager of Heno Bio, and Echo Guo, COO and Co-Founder of 2Firsts, signed the cooperation agreement on behalf of their respective organizations. This collaboration signifies a shared commitment to exploring new opportunities in the NGP market, driving innovation, and promoting sustainable industry growth.

Echo Guo remarked:
“Compliance has become the foundation and essential capability for global industry development. I am grateful for Heno Bio’s proactive efforts in advancing global compliance with us. This collaboration not only supports corporate growth but also sets new benchmarks for compliance innovation, reflecting Heno Bio’s leadership and responsibility as a top-tier company.”

In his keynote speech, Liu Qizhou shared Heno Bio’s compliance practices, emphasizing the importance of upstream compliance, including the application for TPMF (Tobacco Product Manufacturing Facility). He noted that compliance at the source can generate positive, industry-wide ripple effects, further demonstrating the company’s long-term strategy for the entire supply chain.

Taco Tuinstra Joins 2Firsts as Global Editor-in-Chief
2Firsts officially announced the appointment of Taco Tuinstra, former editor-in-chief of Tobacco Reporter, as its new global editor-in-chief. This appointment marks a significant milestone in 2Firsts’ journey toward global expansion.

Since its establishment just three years ago, 2Firsts has rapidly grown into a globally recognized media and consultancy in the NGP sector. With its extensive coverage of major events and conferences, 2Firsts has earned a reputation for providing industry-leading insights. In 2025, 2Firsts will further enhance its global reporting, research, and professional services to promote high-quality development in the global NGP industry.
Interactive Networking and Valuable Insights
The seminar combined both online and offline formats, offering attendees opportunities for real-time interaction. On-site participants could scan QR codes to send live comments and questions, fostering an engaging and dynamic atmosphere. Additionally, the networking lounge provided a relaxed environment for attendees to connect and exchange ideas.
Post-event feedback from participants highlighted the seminar’s immense value in providing actionable insights for navigating global markets. Many expressed gratitude to 2Firsts for creating such a high-quality platform for compliance discussions, which significantly facilitated communication and collaboration within the industry.
As a global leader in NGP media and consultancy, 2Firsts remains committed to building bridges for resource sharing and information exchange. Looking ahead, 2Firsts will continue to integrate resources, unite industry stakeholders, and contribute to the innovative and compliant growth of the global NGP industry.
Agenda Overview
First Session:
- Opening Speech: Alan Zhao, CEO of 2Firsts
- Release of the Top 10 Global NGP News for 2024 – 2Firsts
- Commentary on the Top 10 Global NGP News for 2024 – Taco Tuinstra (2Firsts Global Editor-in-Chief), Li Chen (Analyst, China Cinda Securities Research Institute)
- Analysis and Predictions for Global Market Compliance in 2025 – Azim Chowdhury (Partner, Keller & Heckman)
- Emerging Trends in European NGP Compliance for 2025 – Dr. Yujun Liu (Technical Director, Skyte Testing Technology; PhD, Chinese University of Hong Kong)
- Opportunities and Challenges in PMTA Applications for 2025 – Dr. Jessica Zdinak (Chief Research Officer, CEO, ARAC)
- Insights and Recommendations on Compliance Trends in the Australian Market for 2025 – Frank Lin (Technical Director of E-Cigarette Projects, IMPAQ Testing)
- Opportunities and Challenges in PMTA Applications for 2025 – Dr. Andrea Patton (Ph.D. Behavioural Epidemiologist and Head of Prevalence Research, CSUR)
- Compliance Practices in the Nicotine Industry – Qi Zhou Liu (General Manager, Heno Bio)
- Compliance Action Launch Ceremony Between Heno Bio and 2Firsts
Second Session:
- Release of the 2025 Confidence Index for China’s E-Cigarette Supply Chain Practitioners – 2Firsts
- Insights and Recommendations on Compliance Trends in the Southeast Asian Market for 2025 – Xiaoyan Chen (R&D Manager, Element Testing)
- Insights and Recommendations on Compliance Trends in the Philippine Market for 2025 – Minghui Xiong (General Manager, Webb Testing)
- Emerging Compliance Trends in the Indonesian E-Cigarette Market for 2025 – Garindra Kartasasmita (Secretary-General, Indonesian E-Cigarette Association - APVI)
- The Impact of FDA’s 2024 APPH Science Policy Memorandum on Compliance Development in the E-Cigarette Industry – Dr. Charlene Liu (Founder, President, Chief Scientist, RiskWise Solution)
- Regulations on China’s E-Cigarette Operating Licenses and Compliance-Driven Industry Optimization – Shunliang Tang (Lawyer, Tianyuan Law Firm; Compliance Expert in NGP)
For submissions, interviews, or commentary, contact 2Firsts at info@2firsts.com or connect with CEO Alan Zhao on LinkedIn.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







