
On November 21, US Food and Drug Administration (FDA) released 13 scientific policy memos for reviewing premarket applications for e-cigarettes, spanning from 2020 to 2023. The FDA stated that these memoranda describe the processes and priority methods used for filing and reviewing flavored e-cigarettes and other PMTA applications. ( Read more: "FDA Issues 13 Policy Memos on Flavored E-Cigarette Review Methods").

In light of the memo released, 2Firsts conducted research and consulted with multiple compliance experts and found that the importance of "age gating technology" has been emphasized, meaning devices with age verification will receive priority review. Additionally, some memos also revealed the FDA's additional requirements for flavor studies in later reviews.
Products With Age-Gating Features to Receive Priority Review
A memorandum titled "Filing Prioritization for PMTAs received between September 10, 2020 to November 3, 2021" provides a detailed overview of FDA's policy and background on prioritizing PMTAs that have been received.

In the Memorandum, FDA explained the core principles behind its review prioritization, focusing on two main aspects:
- Submission Date: Applications are generally reviewed in the order they were submitted. For example, a PMTA submitted on September 10 would take priority over one submitted on September 15.
- Special Priorities: Certain applications featuring unique technologies or significant public health benefits may be prioritized. The memos specifically highlighted a PMTA submitted by a company incorporating "age-gating technology." Due to its potential public health impact, this application was prioritized for filing. FDA stated, "The application was prioritized for filing because it contains purported age-gating technology."

"The memos reveal that products with 'age-gating' technology can expedite review, bypass the second round, and proceed directly to the third stage of evaluation," said Kurt, compliance consultant of 2Firsts.
Kurt further explained, "My understanding is that FDA prioritizes reviewing products with age gating features, allowing them to advance directly to the third-stage queue. However, the review standards remain unchanged."
He also noted that this highlights FDA's logic in reviewing vapes: devices with age verification features effectively prevent youth usage and are therefore considered a priority.
Kurt added that such products may not require costly randomized controlled trials (RCTs) or longitudinal cohort studies, as their design inherently addresses youth misuse prevention.
Flavored Products Must Demonstrate "Incremental Benefits"
In a memo titled "ENDS Containing Non-Tobacco Flavored E-Liquid: Approach to PMTAs Not in Substantive Scientific Review (Phase III)," FDA outlined its regulatory strategy for premarket tobacco product applications (PMTAs) for non-tobacco flavored vapes (ENDS).
In the memo, FDA explained its review criteria, citing Section 910 of the Federal Food, Drug, and Cosmetic Act (FD&C Act). A key factor in the review is whether the product is "appropriate for the protection of public health" (APPH). Non-tobacco-flavored vapes must demonstrate "incremental benefits" for adult smokers compared to tobacco flavored products.
"Incremental benefits" require submitters to prove that non-tobacco flavored products offer additional advantages for adult smokers over tobacco-flavored options or other existing alternatives. This assessment requires evidence from randomized controlled trials (RCTs) or longitudinal cohort studies.
Applications lacking such evidence are deemed to have a "fatal flaw", which may result in a Marketing Denial Order (MDO).


Kurt stated that since July 2021, FDA introduced a new requirement for long-term clinical testing for flavored products as part of its review process. This additional requirement led to many companies being deemed non-compliant, with thousands of MDOs issued. Subsequently, numerous lawsuits were filed against FDA, claiming that this requirement violated the original PMTA review standards.
So, how does FDA define "non-tobacco flavors"?
An addendum memo titled "Addendum to Approach to PMTAs for Non-Tobacco Flavored ENDS Not in Substantive Scientific Review (Phase III)" provides answers. It includes a detailed list clarifying which products are considered non-tobacco flavors and defines the scope of "tobacco" and "menthol" flavors.
According to Kurt, the tobacco and menthol flavors outlined in these lists are not subject to flavor-specific reviews. Only FLAVOR SKU undergoes the long-term clinical trial review for potential "fatal flaws." Without supporting evidence, FDA will issue MDO letters for these products.
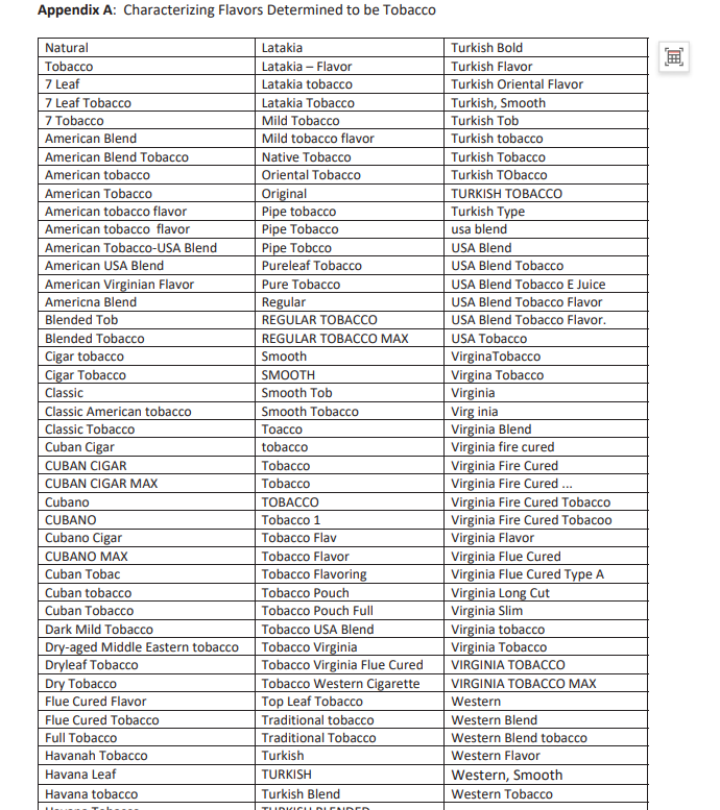
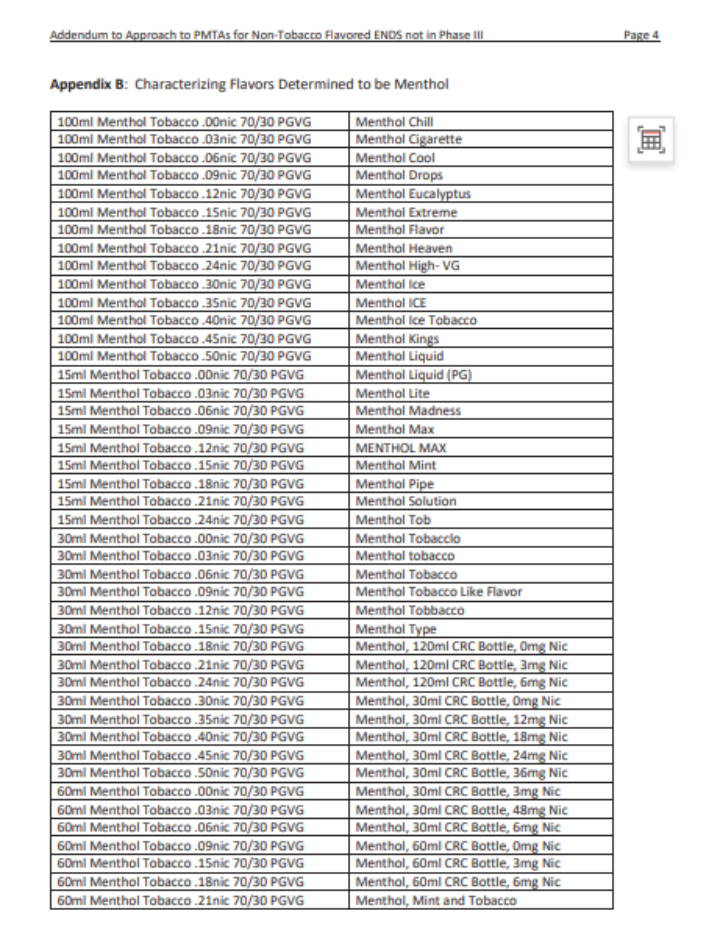
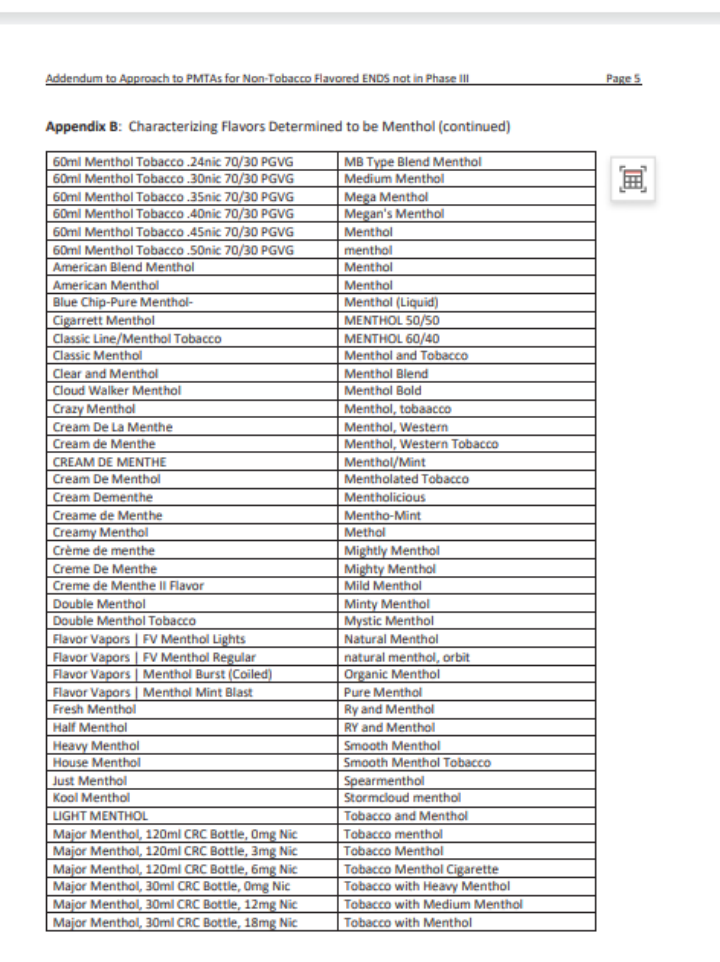
This is the fourth batch of memos released in 2024, bringing the total number of regulatory science policy memos issued by FDA to 26. While FDA states that these memos should not be used as tools, guides, or manuals for preparing or submitting applications, analyzing and interpreting them can still provide deeper and more accurate insights into FDA's review philosophy and approach to vape regulation.
2Firsts will continue to report the latest developments in U.S. vape regulatory policies.
If you have any additional comments, industry insights, or related information regarding this article, feel free to contact us via email at: info@2firsts.com.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








