
On November 7, 2023, U.S. Food and Drug Administration (FDA) announced on its official website that, after a rigorous scientific review, it has renewed the Modified Risk Tobacco Product (MRTP) order for eight snus products from Swedish Match USA, Inc.
With this renewal, these products will continue to be sold (having been approved for sale since 2019) and will carry the following updated risk claim: “Using General Snus instead of cigarettes puts you at a lower risk of mouth cancer, heart disease, lung cancer, stroke, emphysema, and chronic bronchitis.”
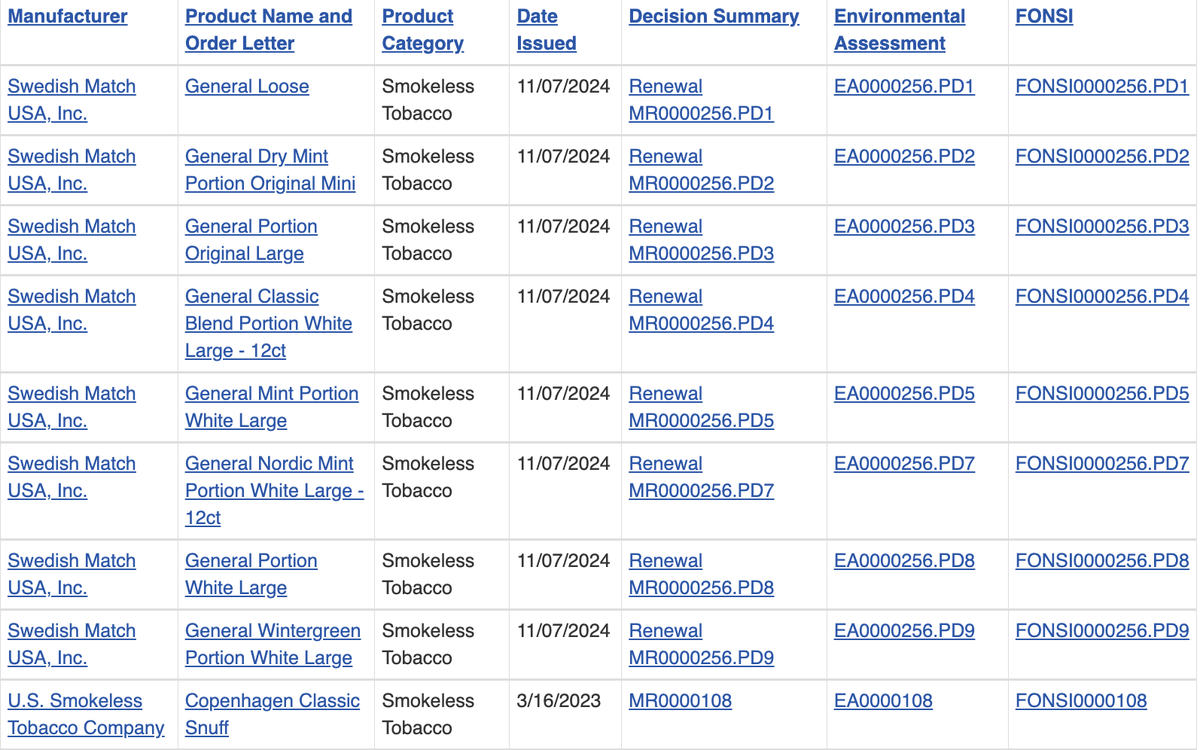
The products granted the risk modification include:
- General Loose
- General Dry Mint Portion Original Mini
- General Portion Original Large
- General Classic Blend Portion White Large-12ct
- General Mint Portion White Large
- General Nordic Mint Portion White Large-12ct
- General Portion White Large
- General Wintergreen Portion White Large
FDA's renewed risk modification order is valid until November 7, 2032. If, at any time, the agency determines that continued sales of these products are no longer beneficial to public health, it may revoke the order.
FDA stated that the review confirmed that the risk-reduction claim is supported by scientific evidence, that consumers understand the claim, and that consumers correctly perceive the relative risk of these products compared to cigarettes. FDA found that when used by consumers, these risk-reducing products significantly reduce harm and the risk of tobacco-related diseases, benefiting overall public health.
Specifically, existing scientific evidence, including long-term epidemiological studies, indicates that using these products in place of smoking can lower the risk of oral cancer, heart disease, lung cancer, stroke, emphysema, and chronic bronchitis. Furthermore, current evidence does not suggest a significant uptake of these products by adolescents.
FDA emphasized that the modified risk order does not permit the company to market these products with any other modified risk claims that might mislead consumers into believing the products are FDA-approved or considered safe by FDA. There is no safe tobacco product, and individuals who do not use tobacco should not begin using these products.
FDA noted that this is the first renewal of a Modified Risk Tobacco Product (MRTP) order.
- These products were originally authorized for marketing in the U.S. via the premarket tobacco product application pathway in 2015
- In October 2019, the products were then authorized to be marketed as modified risk tobacco products.
- Those 2019 orders permitting marketing of General Snus products as modified risk tobacco products were valid for 5 years.
- To continue marketing the products after the set terms of the 2019 orders, Swedish Match USA, Inc. submitted modified risk tobacco product renewal applications.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








