
The US Supreme Court upheld a lower court's decision, rejecting the appeal filed by R.J. Reynolds, a subsidiary of British American Tobacco(BAT), seeking to block the FDA's new graphic cigarette warning labels.
This ruling follows a series of legal battles and leaves in place a decision from the federal appeals court, confirming that the new warnings- featuring images of serious health conditions like cancer, and lung disease- do not violate tobacco companies' First Amendment rights.

Under the Tobacco Control Act of 2009, Congress required that cigarette packaging and advertisements feature "nine new warnings that would rotate regularly", covering "the top 50 percent of the front and rear panels" of each cigarette pack, and at least "20 percent of the area of the advertisement".
In 2011, FDA issued a rule implementing these warnings. However, tobacco companies, including R.J. Reynolds, challenged the rule, arguing it violated their First Amendment rights.
In 2012, the D.C. Circuit Court ruled that the specific images used in FDA's original rule were problematic.
Over the following years, FDA conducted additional research and consumer testing. In 2020, it issued an updated rule with 11 new graphic warning labels based on these findings.
Despite FDA's revisions, R.J. Reynolds and other tobacco companies continued to challenge the 2020 warnings, arguing that they were a form of "compelled speech".They contended that the images would overshadow their messaging. They argued that the "emotional response" evoked by the images was "provocative and misleading".
The Fifth Circuit Court of Appeals upheld FDA's 2020 rule, citing Zauderer v. Office of Disciplinary Counsel (1985), which allows the government to require factual, uncontroversial disclosures in commercial speech for public health purposes.
The court found that the graphic warnings were scientifically accurate, supported by the Surgeon General's report. It also ruled that any emotional reaction to the images did not change their factual content, as their primary purpose was to inform the public about smoking's health risks.
Additionally, FDA emphasized that the warnings are based on "the best available science" and that their primary purpose is to inform the public about the well-established health risks of smoking.
After the Fifth Circuit Court's ruling, the petitioners (the tobacco companies) sought a review by the U.S. Supreme Court, arguing that the lower courts had misapplied the law.
Finally, the US Supreme Court rejected the appeal On 25, November 2024, leaving the FDA's regulation intact. The graphic warnings are set to take effect starting December 2025, 15 months after the final regulations are issued.

FDA's new regulation will be enforced "15 months after the issuance" of the Secretary's regulations, meaning the labels will be displayed on cigarette packaging and advertisements by December 2025.
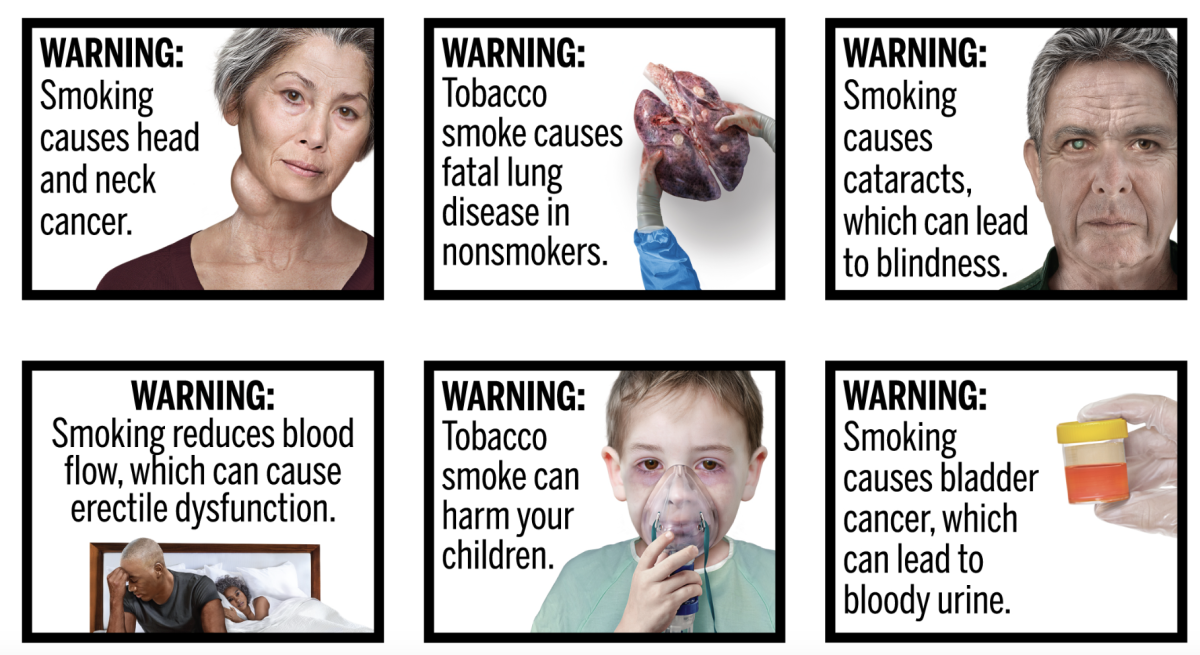
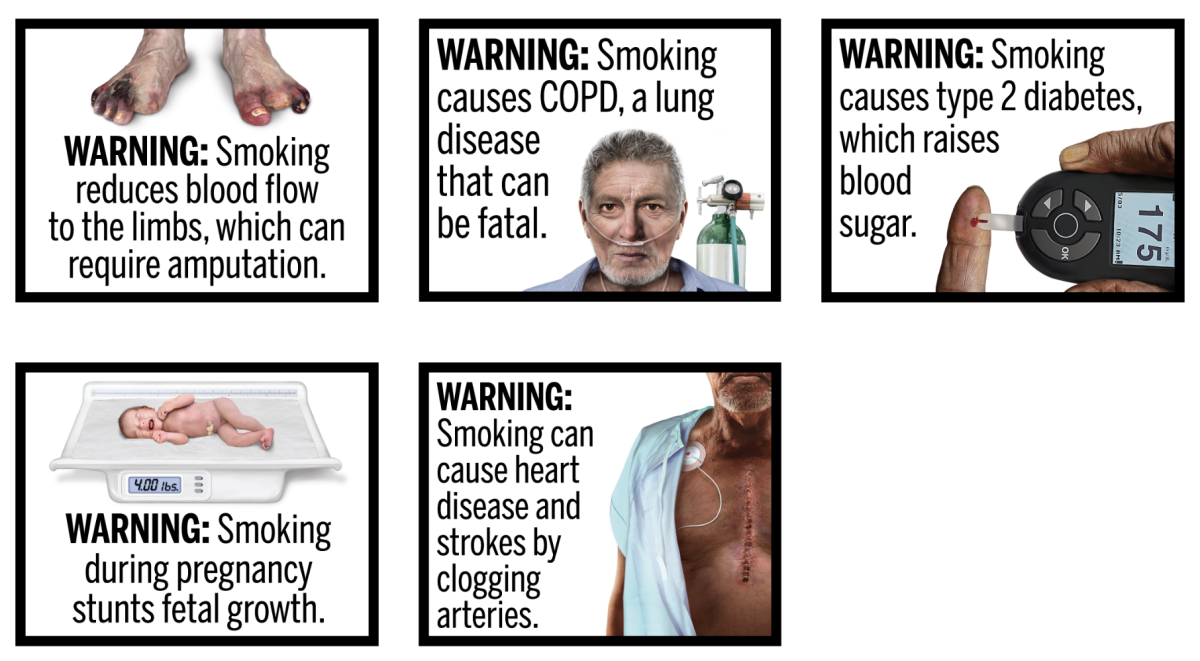
The new graphic warnings include a range of images, such as a woman with a neck tumor and a child with an oxygen mask, both of which convey the "negative health consequences of smoking".








