
According to a report by IPWatchdog on August 15th, the United States Court of Appeals for the Federal Circuit (CAFC) today reversed and remanded the district court's decision to grant a preliminary injunction to VPR Brands, LP for reexamination.
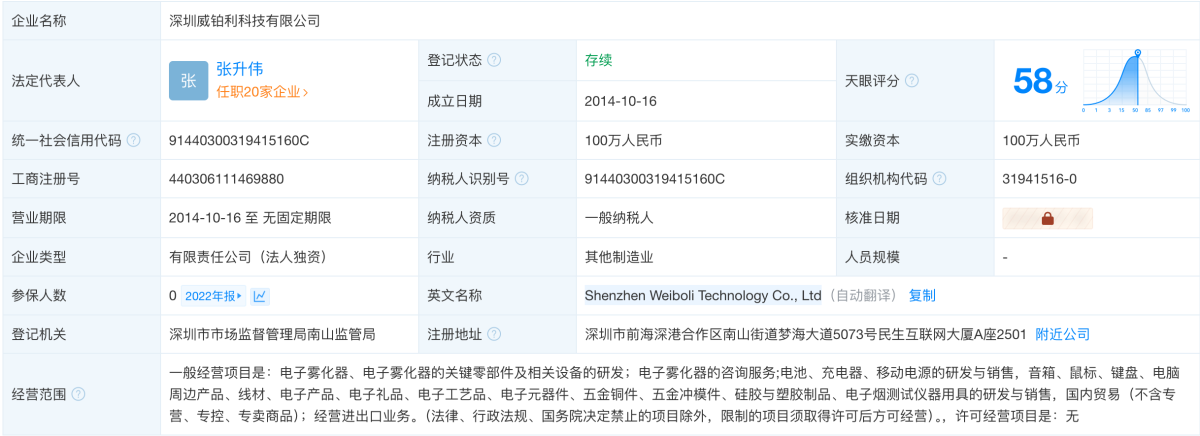
The case began with VPR Brands, LP accusing Shenzhen Waiboli Technology Co., Ltd of infringing on its "ELF" trademark used for e-cigarettes and related products.
Previously, VPR filed a lawsuit against the company Wyvole in the Southern District Federal Court of Florida, alleging that the e-cigarettes distributed by the company under the ELFBAR brand constitute trademark infringement and could cause confusion in the market. The next day, VPR submitted a preliminary injunction application to the court against ELFBAR products.
In response, VPR Corporation countered that, according to the "unlawful use doctrine," VPR's "ELF" trademark is invalid, and VPR will not be successful in its claims of infringement.
According to this principle, the use of trademarks in commerce must be legal. Whaboli Corporation claims that VPR did not obtain pre-market authorization from the US FDA before selling its "new tobacco products," making its use illegal, rendering the trademark registration invalid and unenforceable.
The district court ultimately granted the preliminary injunction, rejecting Wybori Corporation's argument to apply the "fair use doctrine" in the infringement case.
Although the court acknowledged that Viboryl Corporation provided evidence of illegal use under the Food, Drug, and Cosmetic Act (FDCA), the court "refused to analyze Viboryl's evidence to determine if it was sufficient to prove illegal use", and explained that doing so "would require drawing inferences and speculation from the existing record", which is the responsibility of the FDA.
Vibhore subsequently appealed to the Federal Circuit Court of Appeals.
In its appeal, the CAFC stated that...
The district court misunderstood the guidance of the Eleventh Circuit Court of Appeals and unreasonably denied WeiBoli Company's defense of unauthorized use without thorough legal or factual analysis.
The CAFC declined to make a ruling on the evidentiary issue in the first instance, but stated that the grant of preliminary injunction was "not warranted" because the district court erred in interpreting the application of the Eleventh Circuit's principles for illegal use.
The Court of Appeals for the Federal Circuit (CAFC) has explicitly stated that...
Our ruling does not pre-determine whether the district court must adopt the principle of illegal use, or its application in this case.
Our ruling does not prejudge whether VPR's defense of illegal use can be established in the preliminary injunction or subsequent stages in this case. The district court should reconsider all related issues in light of this ruling and reassess VPR's preliminary injunction request.
Therefore, the CAFC has revoked the granting of the preliminary injunction and remanded all relevant issues for further review, including all factors that may have been involved in the granting of the preliminary injunction.
According to Tianyancha, Shenzhen Weipoli Technology Co., Ltd. was established in 2014 with Zhang Shengwei as its legal representative. It is a wholly-owned subsidiary of Heaven Gifts Technology Co., Ltd. The company's business scope includes research and sales of electronic atomizers and their key components, consulting services for electronic atomizers, as well as research and sales of batteries and electronic products.
According to Yahoo Finance, VPR Brands, LP operates in the e-cigarette, electronic cigar, personal vaporizer, and pocket lighter industry in the United States. The company, formerly known as Soleil Capital LP, changed its name to VPR Brands, LP in September 2015. VPR Brands, LP was founded in 2003 and is headquartered in Florida.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








