Charlie's Holdings, Inc. (OTCQB: CHUC, referred to as Charlie's) is a leading company in the high-quality nicotine vapor products industry. The company has recently released its operational data for the second quarter and first half of 2022, highlighting some impressive business performances.
The major financial highlights of Q2 2022 (compared to Q2 2021) are:
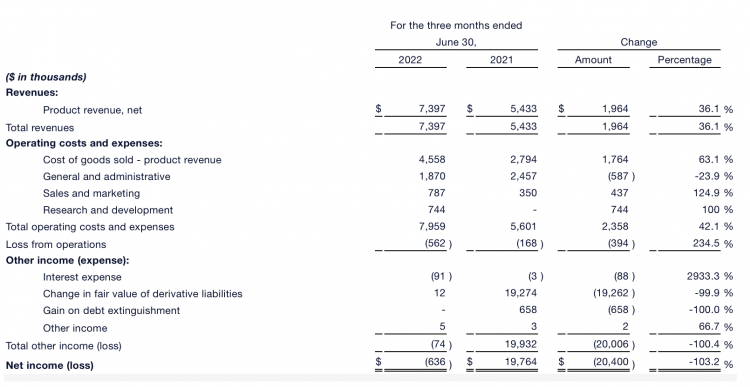
The company experienced a 36% growth in revenue, reaching $7.4 million, and an 8% increase in gross profit, reaching $2.8 million. The percentage of operating expenses decreased from 52% to 46% of revenue. Operating losses, which include $700,000 in research and development costs related to PMTA, increased by $400,000 to $600,000. The net loss was $600,000, while net income was $19.8 million.
The major financial highlights of the first half of 2022 (compared to the first half of 2021) were:
The company saw a 58% increase in revenue, reaching $15.5 million, and a 28% rise in gross profit, hitting $6.5 million. Operating expenses as a percentage of revenue decreased from 56% to 43%. Operating losses, including $800,000 in PMTA-related research and development costs, decreased by $200,000 to $200,000. The net income was $100,000 and the net loss was $400,000.
As of the quarterly operating results for June 30, 2022, compared to the same period last year:
Matt Montesano, the Chief Financial Officer of Charlie's Holdings, has reported that the company's strong momentum has continued into the second quarter and first half of 2022, with revenues increasing by 36% and 58% year-on-year, respectively. "We are continuing to diversify and expand Charlie's powerful product line, as seen in our new 12ml Pacha Syn disposable product line and updated Pacha Syn e-liquid series, both launched in the second quarter of 2022. At the same time, we are operating our business under strict financial controls, with operating expenses as a percentage of revenue further reduced to 46% in the second quarter, demonstrating this commitment.
The Chief Operating Officer of Charlie's, Ryan Stump, stated that their Pre-Market Tobacco Application (PMTA) for 2020 is among the few applications submitted to the FDA that have not received a marketing denial order (MDO) or a rejection file designation. They have invested an additional $700,000 in research and development towards the 2022 PMTA submission in the second quarter of this year to ensure compliance and offer reliable products to their customers. Before the FDA's deadline on May 14, they successfully submitted new PMTA for over 700 Pacha Syn products containing synthetic nicotine SKUs. Stump believes that their extensive investment in R&D and regulatory compliance efforts sets Charlie's apart from most of their competitors' PMTA submissions, putting them in the best position for success.
Charlie's CEO Henry Sicignano III explained, "As we refocus on our product development plans, sales team, marketing strategies, and strategic partnerships, Charlie's is growing. Over the next few months, we plan to launch a series of new disposable e-cigarettes with alternative cannabinoids... as well as new proprietary and innovative cannabinoid products by Charlie's. As a result of all these efforts, I am pleased to report that we are on track for our best year in company history.
Statement:
This article is compiled from third-party information, and is for industry discussion and learning purposes only.
This article does not represent the views of 2FIRSTS, and 2FIRSTS cannot confirm the authenticity or accuracy of the article's content. The translation of this article is solely for the purpose of industry-related communication and research.
Due to limitations in the compiling level, the translated article may not fully reflect the original meaning. Please refer to the original article for accuracy.
2FIRSTS maintains complete alignment with the Chinese government on any domestic, Hong Kong, Macau, Taiwan, or foreign-related statements and positions.
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.







