According to a Reuters survey, disposable flavored e-cigarettes, which are usually sweet-tasting, account for one-third of the U.S. e-cigarette retail sales, up from nearly 2% three years ago. The survey results show that consumers have spent more than $2 billion on e-cigarettes in the past year.
Reuters has obtained survey results from Chicago-based market research company IRI, which uses scanner-recorded data and other information to track retail purchases. The IRI data measured purchasing behavior between January 12, 2014 and June 12, 2022, and included observations on Altria Group Inc., which holds a 35% stake in Juul Labs Inc. following FDA action against the company.
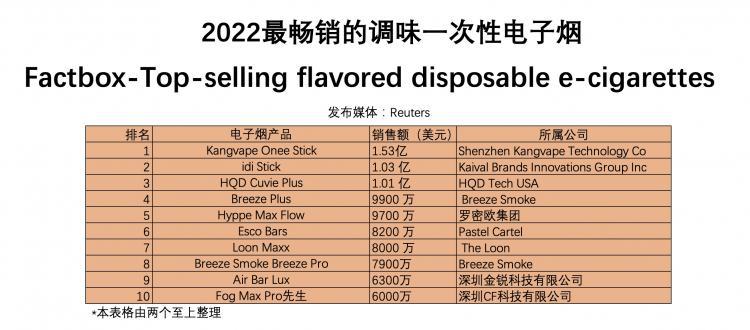
The data was shared with Reuters by an anonymous individual outside of IRI. A spokesperson for IRI stated that the company "cannot confirm any information received from external sources" and that these data should not be attributed to IRI. According to annual estimated sales figures from June 20, 2021, to June 12, 2022, the following are the top-selling disposable seasoning brands and their parent companies in the United States.
Statement
This article is based on compiled third-party information and is intended for industry-related communication and learning purposes only.
This article does not represent the views of 2FIRSTS, and 2FIRSTS is unable to confirm the authenticity and accuracy of the article's content. The translation of this article is only intended for communication and research within the industry.
Due to limitations in translation ability, the compiled article may not completely reflect the original text. Please refer to the original text for accuracy.
2FIRSTS maintains complete alignment with the Chinese government's stance on any domestic, Hong Kong, Macao, Taiwan, or foreign-related statements and positions.
The copyright of compiled information belongs to the original media and authors. If there is any infringement, please contact us for deletion.
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.







