
Disclaimer: The content of this article represents only the original media's position, 2Firsts only reprints it and does not represent the views of our platform.
According to representatives from regulatory agencies in the United States and two companies, some e-cigarette companies targeted by US authorities have changed their business models or company structures, including transferring operations to offshore companies.
The US Food and Drug Administration (FDA) stated that such tactics make efforts to prevent unauthorized e-cigarettes from entering the US more complicated.
The FDA stated in a press release to Reuters that they are aware and closely monitoring situations where some companies are attempting to change the labeling or business structure of illegally sold products in order to evade detection.
The regulatory agency did not comment on specific brands or companies.
Chinese e-cigarette giant Heaven Gifts revealed to Reuters that after the FDA banned several companies in China, the US, and South Korea from importing its flagship brand Elfbar in 2023, the company transferred its brand Lost Mary's ongoing operations in the United States (products of this brand are not authorized in the US) to a British Virgin Islands (BVI) company Wonder Ladies Limited. Lost Mary continues to be widely sold in the US.
The company's website states that Ludicrous Distro, an e-cigarette company based in Texas (trading under the name American Vape Company), has halted the sale of its own unauthorized Esco Bar brand e-cigarettes and is instead distributing various unauthorized devices from third parties.
Heaven Gifts spokesperson Jacques Li stated that following the FDA's ban on Elfbar, the Chinese company has transferred its operations in the United States from Lost Mary to Wonder Ladies, effectively exiting the US market, and they are "not circumventing FDA regulations".
When asked why they were selling unauthorized devices through their website, Ludicrous Distro refused to disclose the reasons for their change in practice, but told Reuters that they have been working to comply with the ever-changing FDA policies, as these policies have caused confusion.
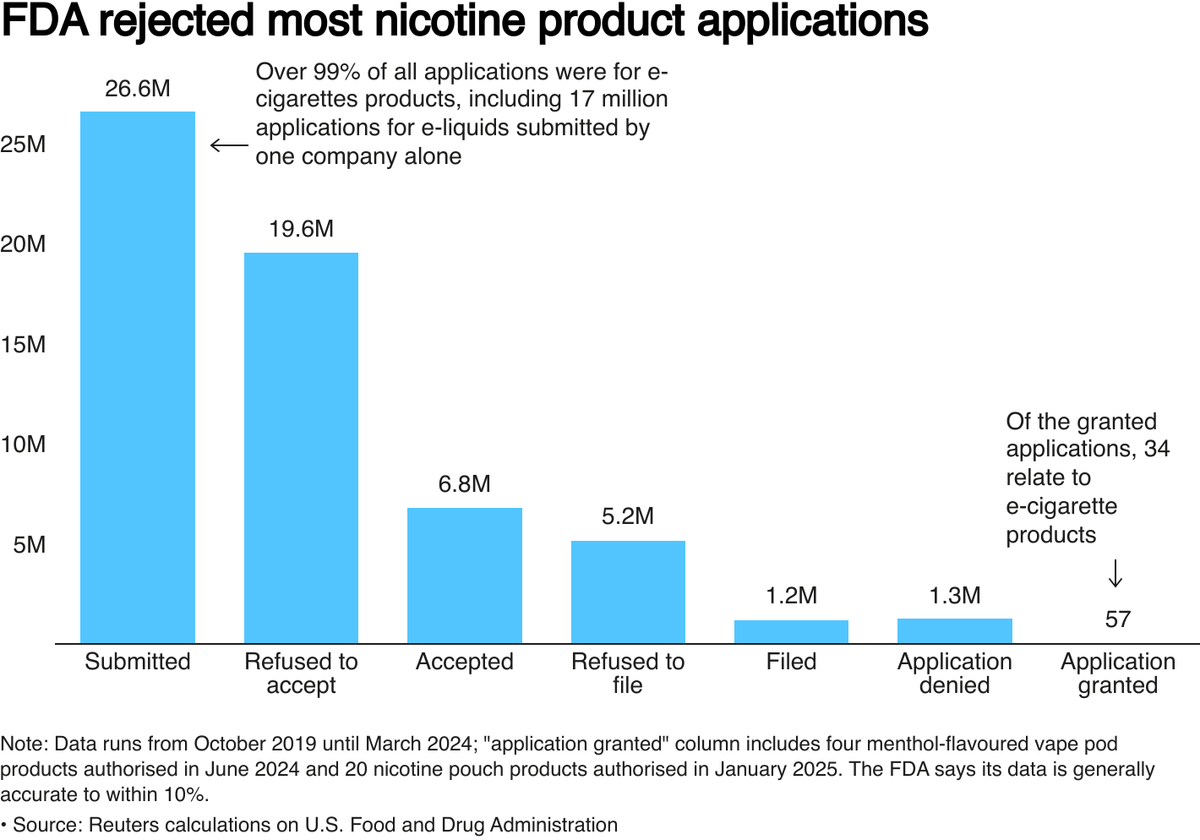
According to data from the FDA, the agency responsible for regulating tobacco and alternative products, since October 2020, approximately 26 million new nicotine devices have been denied licenses, particularly flavored e-cigarettes that are said to be appealing to teenagers.
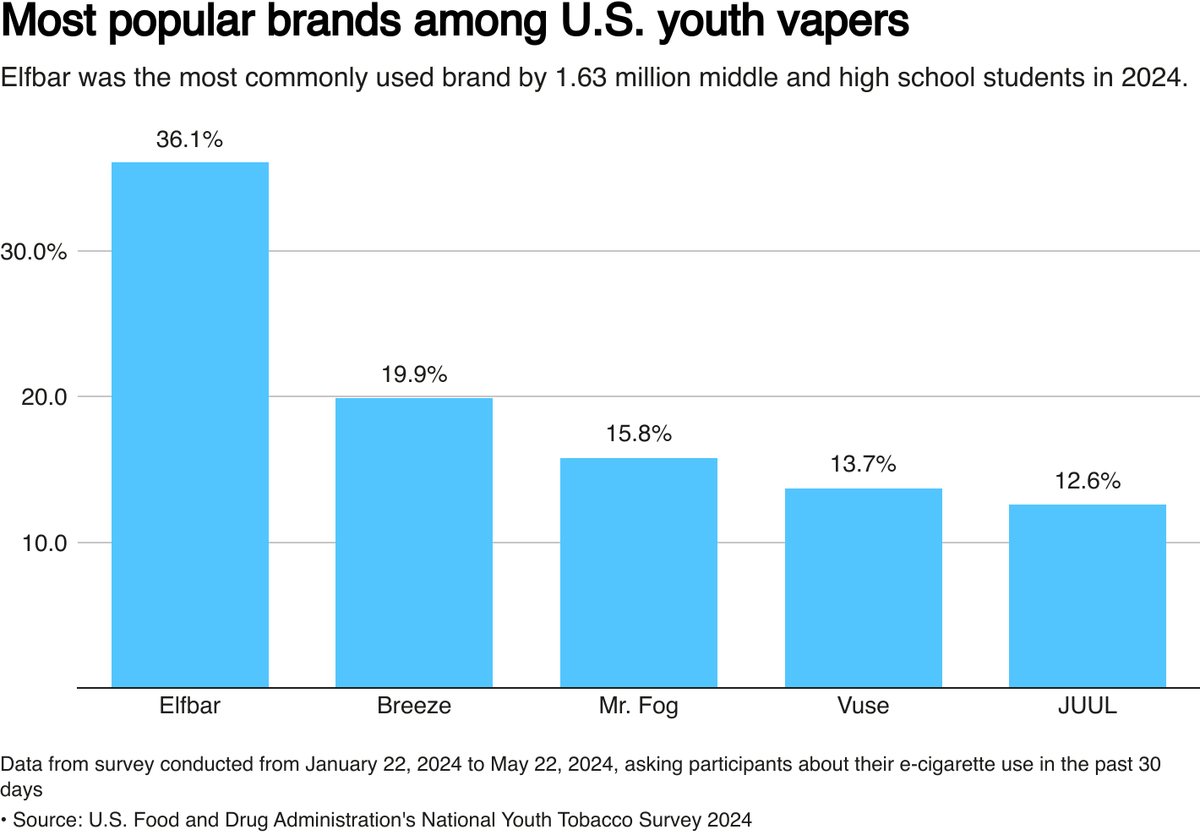
Some unauthorized products, including Elfbar, Lost Mary, and Esco Bar, which are legal in other countries, are being widely sold in the largest e-cigarette market in the world - the United States.
According to a 2023 report by Reuters, Zhang Shengwei's company Heaven Gifts, with the support of Elfbar, has captured over 9% of the e-cigarette market in the United States in the year leading up to September 2023.
Market research company Circana's data shows that Lost Mary is the seventh most popular disposable e-cigarette device in 2024. The data was provided by industry insiders and verified by Reuters. Circana stated that they are unable to confirm or comment on non-public data.
Li refused to clarify the specific terms or timeframe for transferring business to a British Virgin Islands company.
According to the registration records of the United States Patent and Trademark Office (USPTO) as of February 2025, Heaven Gifts still owns the Lost Mary trademark in the United States through its Hong Kong subsidiary, Imiracle (HK) Limited.
However, equipment purchased by Reuters in December shows that the name of Wonder Ladies and an address in the British Virgin Islands now appear on packaging for Lost Mary in the United States.
Reuters reviewed company registration information and found that Tu Xiaoliang, a director of Imiracle (HK) Limited, is the authorized representative for Wonder Ladies and three other British Virgin Islands companies in Hong Kong. These companies share the same office building. Documents show that Tu Xiaoliang is one of the three directors of Imiracle (HK) Limited, with the other two directors being the founder Mr. Zhang.
Wonder Ladies and three other British Virgin Islands companies either own trademarks in the United States or appear on the packaging of unauthorized e-cigarette brands Lost Mary, off-Stamp, RabBeats, Funky Lands, and Quasar.
Tu did not respond to detailed questions sent to its corporate address by Reuters. Four British Virgin Islands companies also did not respond to letters seeking comment sent to their Hong Kong office addresses.
Li stated that he does not know the relationship between Tu and the British Virgin Islands company, and he is unable to explain it. Due to privacy concerns, he declined to disclose Tu's role at Heaven Gifts.
Richard Marianos, former deputy director of the Bureau of Alcohol, Tobacco, Firearms and Explosives, stated that companies in the British Virgin Islands can help to create distance between e-cigarette brands and manufacturers, while also protecting their revenue from law enforcement interference.
A former employee who had direct knowledge of the matter also told Reuters that Heaven Gifts set up a British Virgin Islands company to distance itself from the ongoing illegal sales of Lost Vape products in the US and other unauthorized labels found by Reuters.
The anonymous source implementing this strategy for the Chinese e-cigarette company requested to remain anonymous out of fear of legal repercussions. The source did not provide any written evidence. Reuters was unable to independently verify the source's claims.
Reuters is unable to confirm the ownership of Wonder Ladies, whether the British Virgin Islands company is earning revenue from sales of Lost Mary in the United States, or if revenue is flowing from this British Virgin Islands company to Heaven Gifts.
Confused?
According to data from the US Patent and Trademark Office, Ludicrous Distro has been relying on Chinese manufacturers to produce Esco Bar in recent years and holds the trademark for the brand.
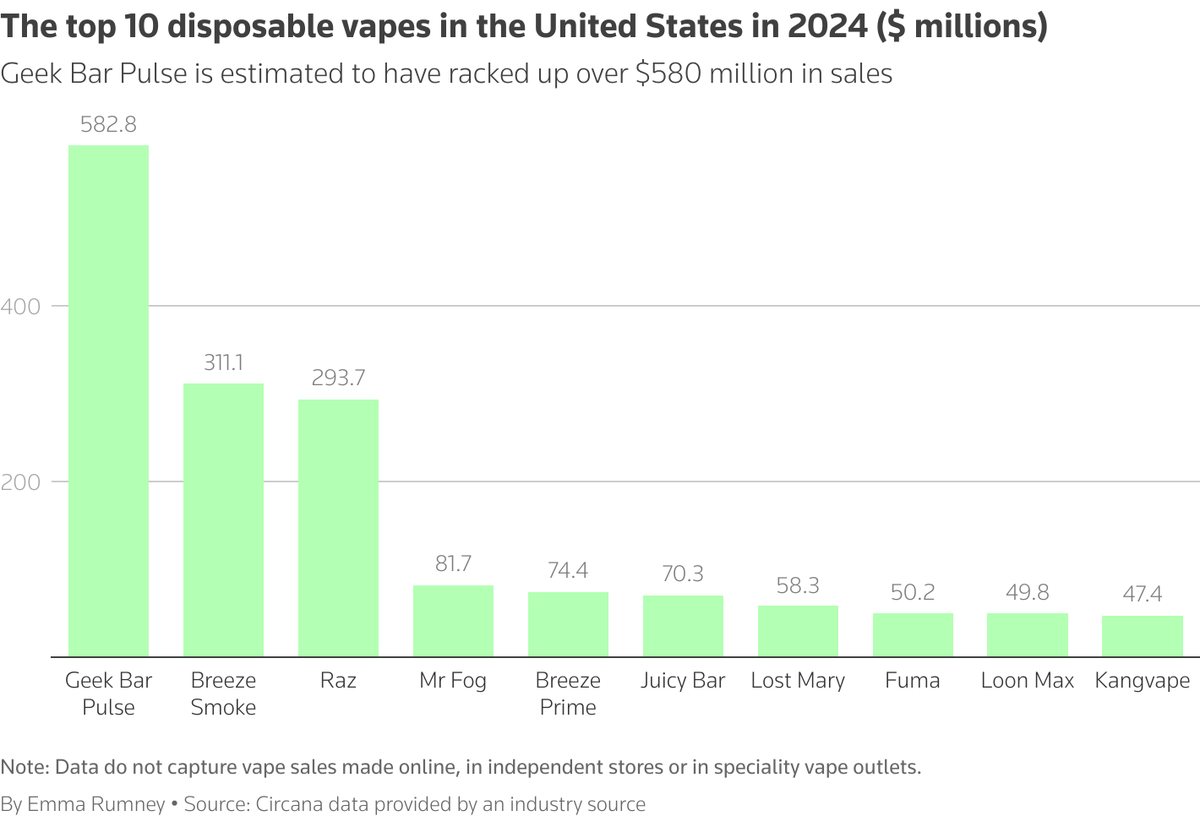
However, the lawyer for Ludicrous Distro told Reuters that the company ceased distribution of the Esco Bar after it was placed on the FDA's red import alert list in May 2023. Esco Bar was the sixth most popular disposable e-cigarette flavor in the United States in the year leading up to June 2022. The lawyer also stated that Ludicrous Distro now only operates as a distributor for third-party e-cigarettes and a range of other products.
The lawyer also stated that this transition "was carried out in a transparent and legal manner", and Ludicrous Distro also "confessed its business operations to FDA inspectors".
An inspection of the Ludicrous Distro website in the United States reveals that the company is offering dozens of unauthorized devices, including seven labels identified as illegal in warning letters and press releases issued by the FDA to retailers, such as Geek Bar and Raz.
Ludicrous Distro stated that many unauthorized products, including those from major tobacco companies, are still being sold on the market without any warnings from the FDA. This has caused significant confusion among consumers about which products are legally available for sale.
The enforcement efforts of the FDA are facing challenges, including a lack of funding, but former director of the FDA Center for Tobacco Products Mitch Zeller stated that any unauthorized e-cigarettes introduced in recent years are "completely illegal." As part of President Donald Trump's efforts to reduce the federal workforce, some FDA staff have recently been laid off.
So far, the FDA has only approved 34 tobacco and mint-flavored e-cigarette products, all of which are produced by large tobacco companies including British American Tobacco (BAT).
Utilizing global awareness
The American Vapor Manufacturers Association stated that the FDA's reluctance to approve flavored e-cigarettes is preventing smokers from accessing legal products that could help them quit smoking. Three American smokers told Reuters that these e-cigarettes helped them quit smoking.
In 2023 and 2024, British American Tobacco (BAT) claimed that illegal devices were eroding its market share in the United States. BAT launched two complaints with the U.S. International Trade Commission (ITC) targeting dozens of e-cigarette brand owners, manufacturers, and distributors.
British American Tobacco (BAT), accused of patent infringement and unfair competition, includes another subsidiary, iMiracle (Shenzhen) Technology Co., Ltd, as well as the American e-cigarette company (officially known as Ludicrous Distro). BAT CEO Tadeu Marroco stated on February 13 that BAT had withdrawn a complaint filed in January, with plans to refile a stronger lawsuit later.
The proposed tax on Thursday only applies to products containing nicotine, including pod-style and cartridge-style refills, disposable e-cigarettes, and bottled e-liquids. The tax appears to include nicotine base sold for DIY purposes. It does not apply to hardware that does not contain e-juice.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








