
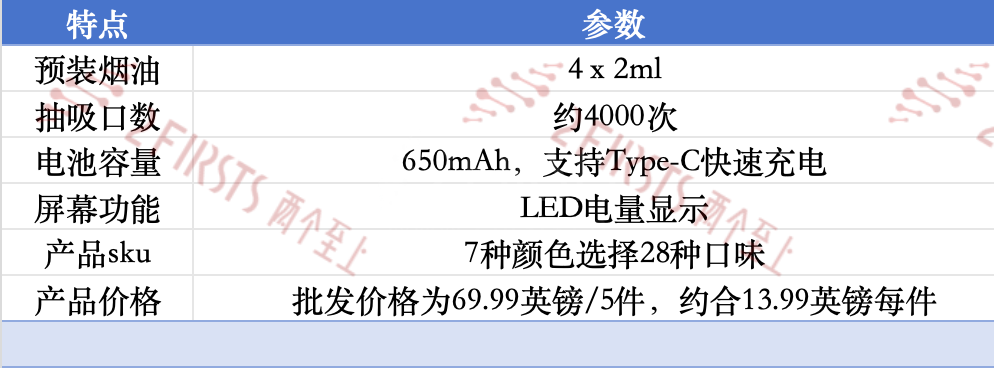
Recently, 2Firsts noted that FUMOT's new 4-in-1 product, the Fumot Tornado 4in1, has been listed on the brand's official website as well as on UK e-cigarette distributor sites like Vapesourcing and Powervapeshop. The product complies with TPD standards and offers approximately 4,000 puffs.

Specifically, the features of the Fumot Tornado 4in1 include:
At the InterTabac tobacco exhibition held in September, the FUMOT brand was interviewed by the 2Firsts team. Brand representatives said, "New large disposable products that meet TPD standards are emerging," and emphasized the importance of actively complying with regulatory standards for long-term stability. The brand also launched several innovative products, such as "2+10," at the exhibition to meet the demands of the German market.
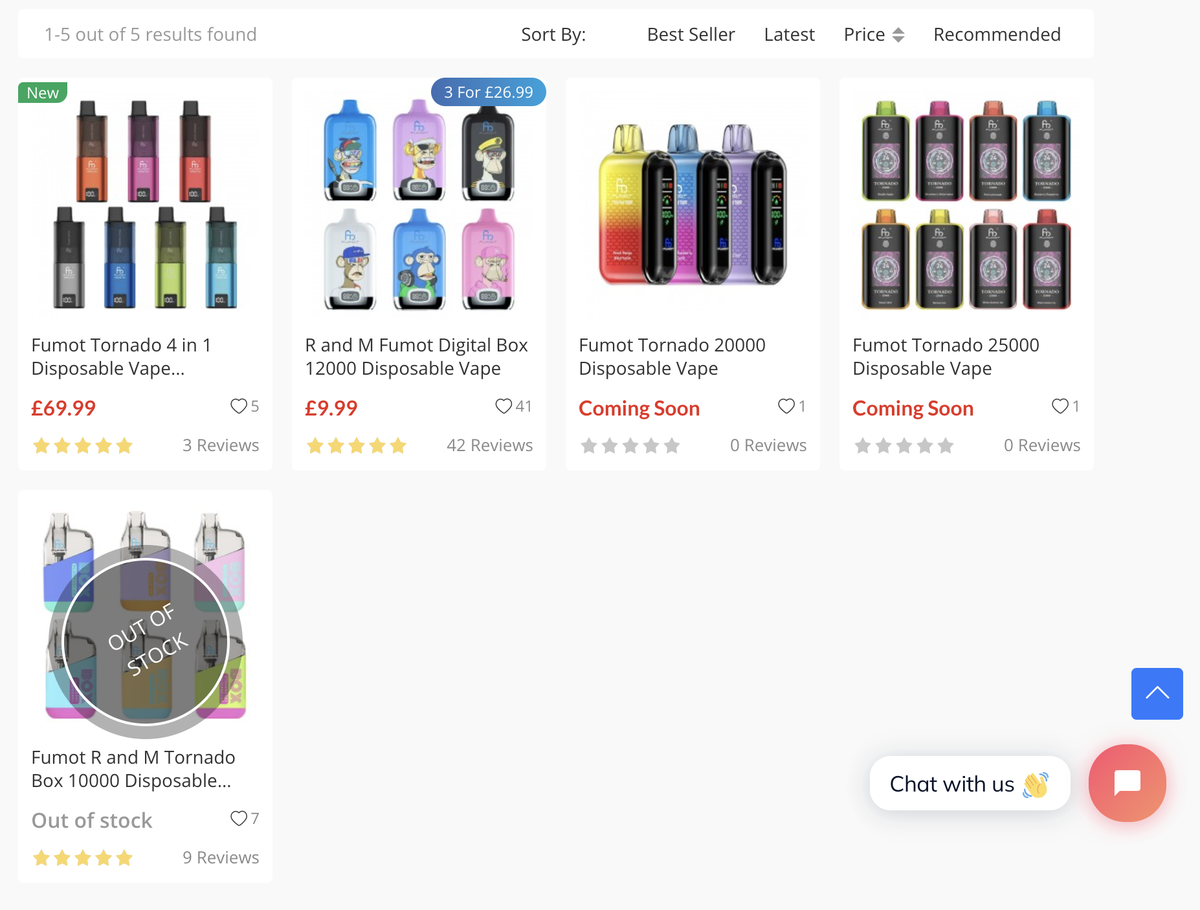
According to observations by 2Firsts, websites that offer online sales of FUMOT e-cigarettes in the UK are relatively limited. FUMOT products were not found on popular sites such as VapeUK, VapeClub, eCigaretteDirect, and ZeroVapes. However, on sites like Vapesourcing and PowerVapeShop, where FUMOT products are available, the offerings mainly consist of high-puff disposable e-cigarettes.
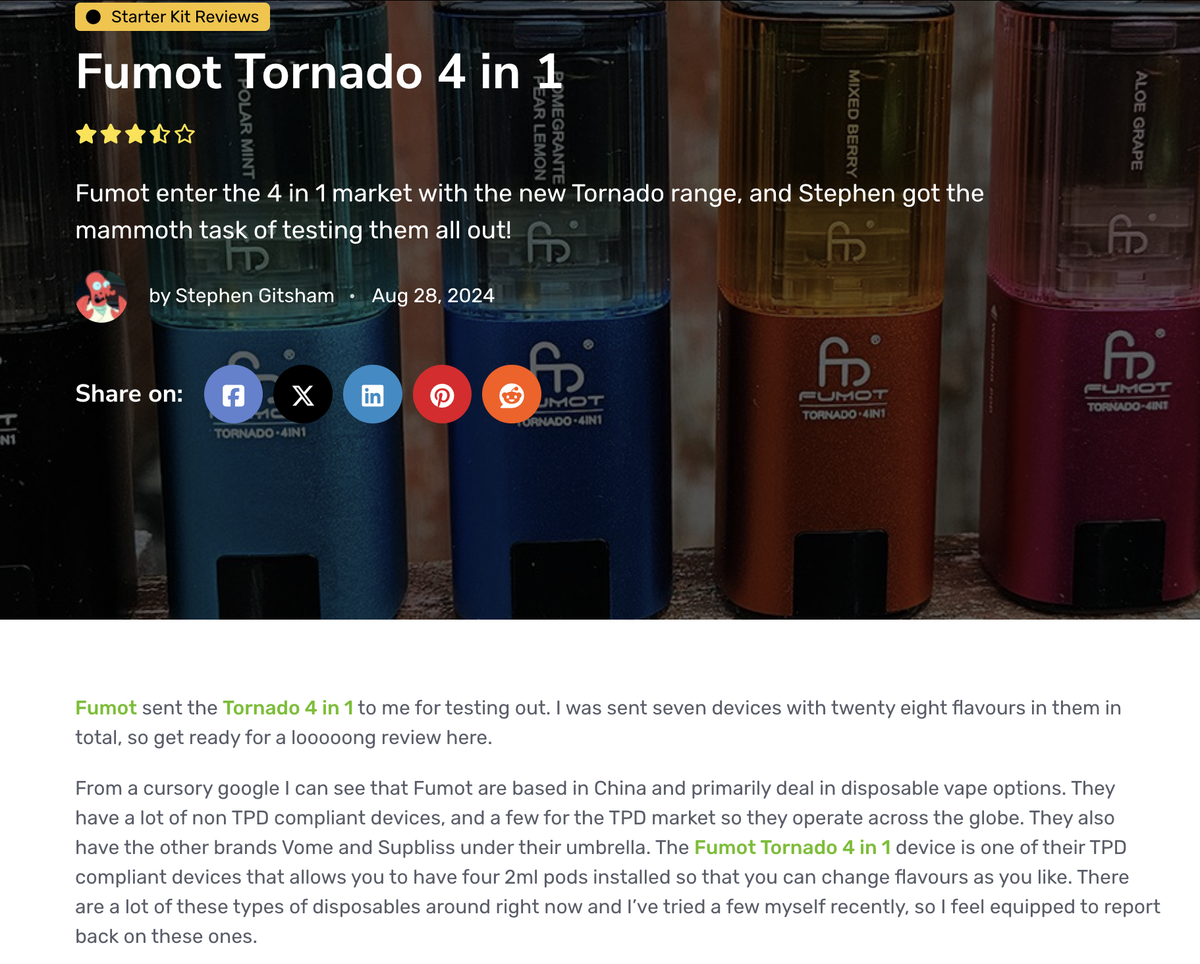
In a review article on the Fumot Tornado 4in1 by the e-cigarette website *Planet of the Vapes*, it was noted: “They [referring to the FUMOT brand] have many devices that do not comply with TPD (Tobacco Products Directive) regulations, but also a few that do, which is why their business is global.”
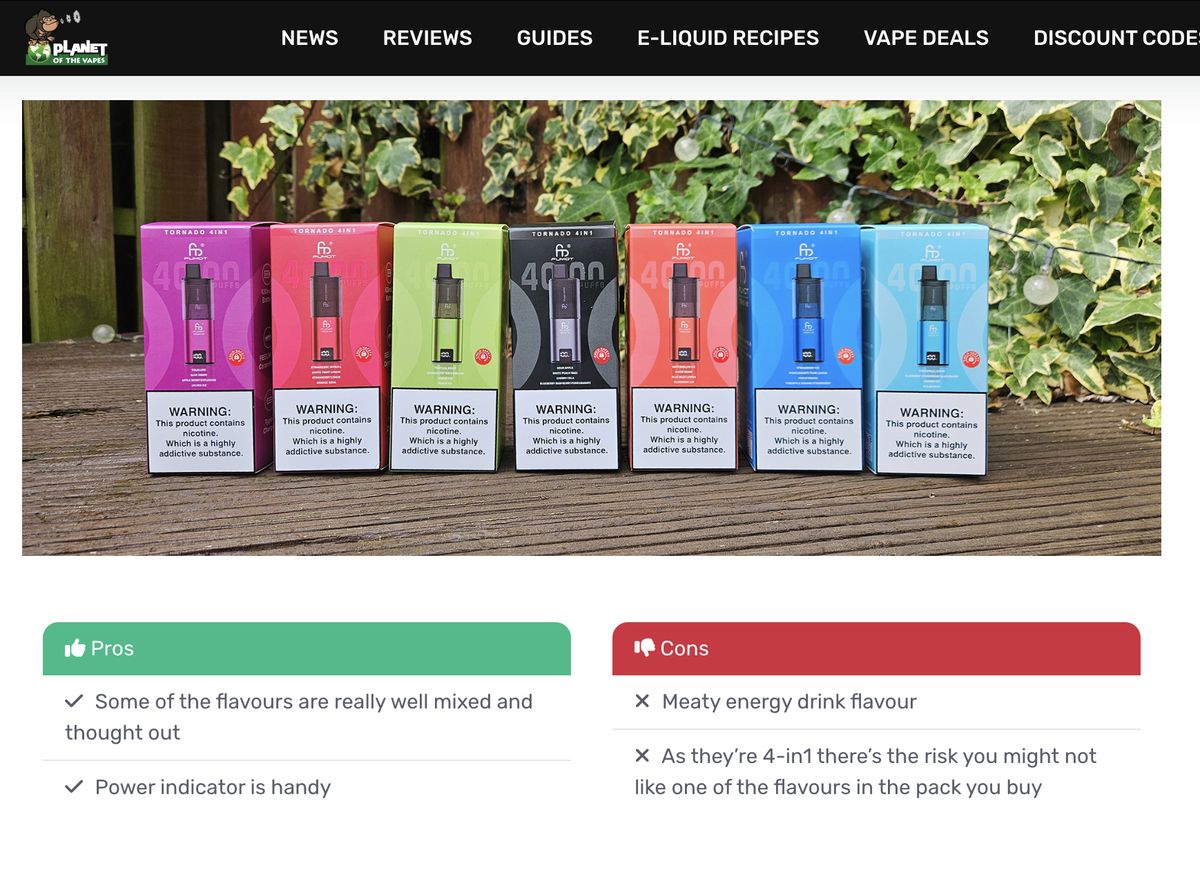
“Some of the flavors [of the Fumot Tornado 4in1] are indeed well-blended.”
“Since they are 4-in-1, you might find a flavor in the pack you purchase that you don’t enjoy.”
It is worth noting that on FUMOT’s official website, a separate “TPD Series” section is listed under the product category, featuring products compliant with TPD standards. This series includes low-puff disposables, open systems, refillable models, and 4-in-1 multi-flavor devices.
2Firsts will continue to monitor the latest e-cigarette products and market trends globally, providing the public with accurate and authoritative industry information.
2Firsts' product column remains committed to delivering readers the latest product updates in the new tobacco field. We invite readers to submit insights and news on e-cigarette developments.
For unique perspectives or information, feel free to contact us at carrie.cai@2firsts.com or scan the QR code below.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







