
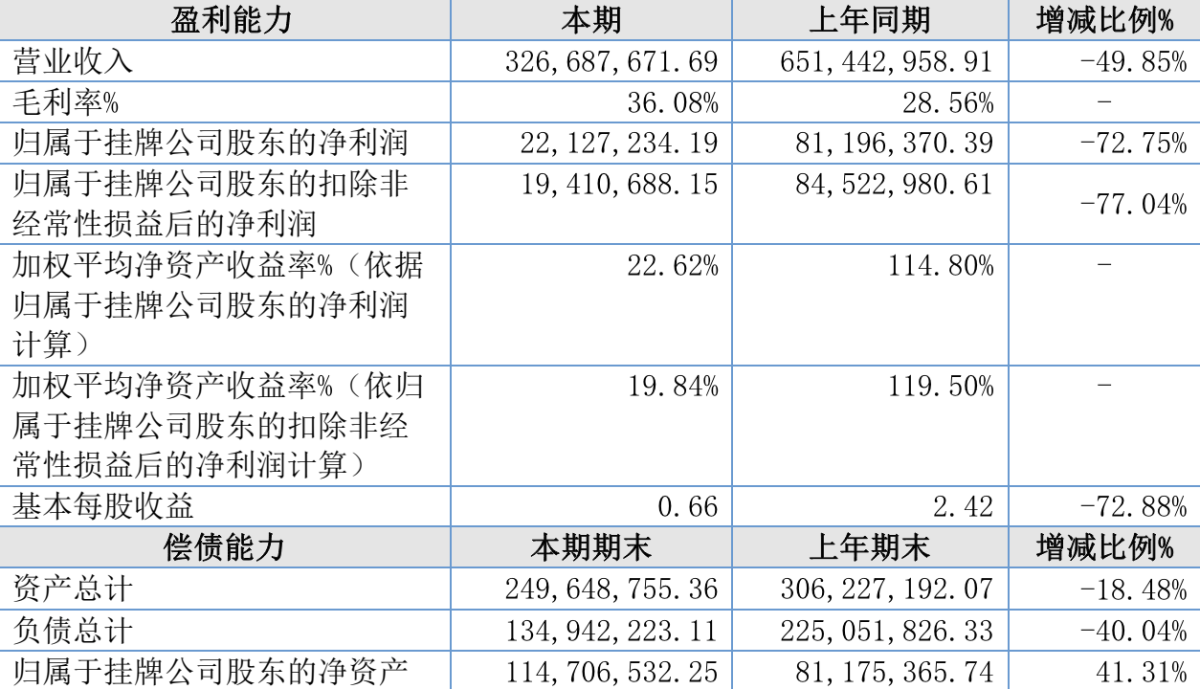
On April 26th, Itsuwa released its 2023 annual report. During 2023, the company's operating income was 327 million yuan, a decrease of 49.85% compared to the previous year. Net profit attributable to the listed company's shareholders was 22.1272 million yuan, a decrease of 72.75% year-on-year.
The Itsuwa report shows that the company primarily employs an "ODM/OEM production and designated sales" business and sales model, supplying e-cigarettes and their related products to overseas e-cigarette brands for revenue, profit, and cash flow.
According to the Itsuwa report, the main reasons for the decline in income are: the impact of e-cigarette policies, the domestic ban on the sale of flavored e-cigarettes other than tobacco flavor, leading to increased competition in overseas markets.
The company's main operating gross profit margins in 2021, 2022, and 2023 were 26.20%, 28.56%, and 36.08% respectively. The gross profit margin in 2023 increased, primarily due to the appreciation of the US dollar exchange rate, an increase in the selling price converted to Renminbi compared to the same period last year, and a decrease in prices of key upstream raw materials such as batteries and microphones.
It is worth noting that the report highlights that their research and development products include disposable e-cigarettes, pod-system e-cigarettes, with a focus on environmental protection, modularity, and multiple pods.
- The company has developed a biodegradable disposable e-cigarette product in compliance with environmental regulations. The product features a biodegradable shell and a modular design, allowing consumers to return it to designated dealers after use. Dealers can then disassemble the product into biodegradable shells and rechargeable batteries, which can be processed back into disposable e-cigarette products. This product is easy to assemble, has a biodegradable shell, and allows for the reuse of batteries, making it environmentally friendly.
- In order to improve the user experience, the company has developed a line of multi-pod products. These pods can be easily interchanged with different flavors and feature a unique rotating structure for convenient and quick switching.
Since 2017, the company has actively promoted its own brands of e-cigarettes, such as VAPESOUL and VOOM, targeting different consumer groups with each brand. The sales of own-brand products account for 23.39% of total sales in this period. In 2024, the company plans to continue promoting the sales of its own brand e-cigarettes.
Itsuwa mentioned that the two actual controllers of the company, due to the difficulties in business operations in 2023 compared to the previous year (sub-company Shenzhen Wudian Technology Co., Ltd. did not obtain a tobacco monopoly license, resulting in the cessation of e-cigarette business operations, reducing the overall e-cigarette production capacity of the company, and the short-term benefits of its business transformation could not be achieved), have voluntarily waived their personal bonuses for the 2022 fiscal year amounting to 4,999,110.00 yuan in order to alleviate the company's financial pressure.
Itsuwa stated that, based on the industry's current situation, regulations are becoming stricter, competition is intensifying, and companies are facing increasing challenges.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







