
Special Announcement:
This article is only for industry professional internal research and exchange, no brand, or product recommendation; minors are prohibited from reading.
JUUL's chief product officer, Kirk Phelps, made a statement:
"Our company DNA is product innovation. With our next-generation platform, we have designed a technological solution for two public-health problems: improving adult-smoker switching from combustible cigarettes and restricting underage access to vapor products. This is only the beginning of new tech being developed and refined for the U.S. market and abroad to eliminate combustible cigarettes and combat underage use."
Launching as early as 2021 in the UK, this latest e-cigarette platform, called the JUUL2 System, is said to offer a better experience for adult smokers, employing a unique authentication technology to tackle illegal products and featuring age verification technology.

Based on feedback from adult smokers, features of the next generation platform include:
- A more consistent vapor experience that better competes with combustible cigarettes
- A Bluetooth-enabled device with a larger, long-lasting battery and a “smart light system” that communicates battery life and e-liquid level to the user
- Newly-designed, tamper-resistant pods that enable improved aerosol delivery
- An innovative heating element that improves product performance and temperature-control precision
- A unique Pod ID chip that, among other tech capabilities, prevents the use of illicit counterfeit and compatible pods with the next-generation device
- A mobile and web-based app that enables age-verification technology, including device-locking, and real-time product information and usage insights for age-verified consumers with industry-leading data-privacy protections
JUUL describes that initial behavioral research with the new platform in the UK has shown compelling switching and use among adult smokers. Six months after purchasing the product, more than 32 percent of JUUL2 System users had completely given up combustible tobacco.
And while the JUUL System, as currently sold, has successfully converted more than 2 million adult U.S. smokers, the company hopes to bring this new technology to the more than 28 million adults in the U.S. who continue to use combustible tobacco, a leading cause of preventable death.
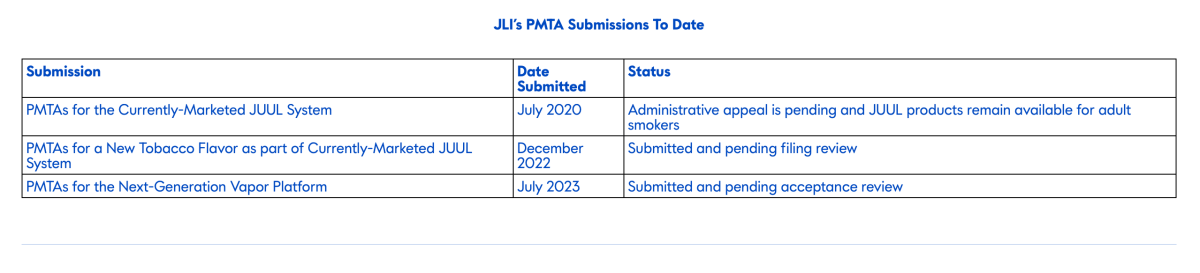
Additionally, the company is continuing its administrative appeal of the FDA's decision to approve the JUUL System and believes that the product will not be subject to political interference under decisions of science and evidence, and that the product will receive marketing authorization.
Finally, JUUL has attached its submitted PMTA, but none of its products have yet passed the review application.
Reference:
*The content excerpted or reproduced in this article comes from a third-party, and the copyright belongs to the original media and author. If any infringement is found, please contact us to delete it. Any entity or individual wishing to forward the information, please contact the original author and refrain from forwarding directly from here.

