
On July 23, Philip Morris International (PMI) (NYSE: PM) released its performance report for the second quarter and first half of 2024. The report revealed that the shipment volume of the US ZYN nicotine pouch reached 135.1 million cans, an increase of 50.3% compared to last year. The shipment volume of canned oral smoke-free products (SFP2) also grew by 23.5%, indicating rapid growth in this segment of the market.
2FIRSTS analyzed the market performance of Philip Morris International's (PMI) ZYN nicotine pouches over the past year, aiming to provide an in-depth analysis of the market prospects for oral smokeless products, particularly nicotine pouches.
(1) Overview of the growth of ZYN nicotine pouch sales in the United States.
It is evident from PMI's 2024 Q2 and first half performance report that ZYN nicotine pouches, as a smokeless nicotine product, have garnered high acceptance among American consumers, with this type of product already capturing a significant market share in the US market.
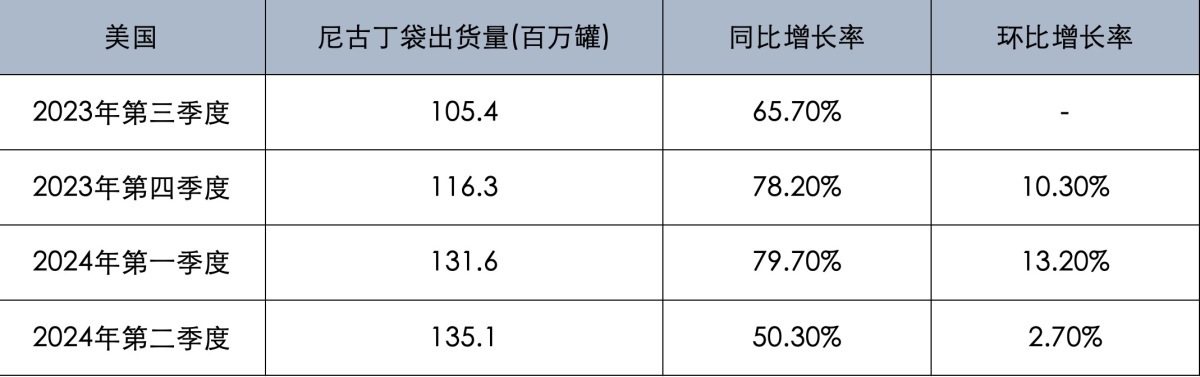
Over the past four quarters, the shipment volume of nicotine pouches has consistently maintained a year-on-year growth rate of 50%.
In particular, the shipment volume of ZYN nicotine pouches in the United States reached 135.1 million cans in the second quarter of this year, an increase of 50.3% year-on-year; in the first quarter of this year, the shipment volume of ZYN nicotine pouches in the US also reached 131.6 million cans, an increase of 79.7% year-on-year.
In the first half of 2024, the total shipments of ZYN nicotine pouches in the United States reached 266.7 million cans, with the total shipments of canned oral products reaching 486 million cans.
In the fourth quarter of 2023, the sales volume of ZYN nicotine pouches in the U.S. was 1.163 billion cans, a 78.2% year-on-year increase; in the third quarter of 2023, the sales volume of ZYN nicotine pouches in the U.S. was 1.054 billion cans, a 65.7% year-on-year increase.
From the third quarter of 2023 to the first half of 2024, ZYN nicotine pouches have experienced four consecutive quarters of growth in the US market. Although the year-on-year growth rate and quarter-on-quarter growth rate decreased in the second quarter of 2024, overall growth momentum remains strong, with shipment volumes of nicotine pouches increasing by 63.5% compared to the first half of 2024.
(2) Overview of ZYN nicotine pouch sales in other regions in the past year.
In the second quarter of 2024, nicotine pouch sales outside the United States increased by over 50%, with new markets such as Pakistan performing well. In Europe, the shipment volume of nicotine pouches reached 11.8 million cans, a year-on-year increase of 26.9%. In the first half of 2024, the total shipment volume of nicotine pouches in Europe reached 24.1 million cans, a year-on-year increase of 40.2%.
In the fourth quarter of 2023, the shipment volume of ZYN nicotine pouches in the Scandinavian Peninsula (including Norway and Sweden) increased by 3.0% compared to the same period in 2022, with an annual shipment volume growth of 6.1%. In the third quarter of 2023, the shipment volume of ZYN nicotine pouches in the Scandinavian Peninsula also reached 7.5 million cans.
(3) Analysis of the market outlook for nicotine pouches.
By analyzing the market performance of ZYN nicotine pouches over the past year, it is evident that smoke-free nicotine products have significant market potential, especially in the global and US markets.
According to PMI's forecast, the shipment volume of ZYN nicotine pouches is expected to reach 560-580 million cans in 2024. Currently, the completion rate of this process is about 47%, and PMI also stated that they will further invest in ZYN to increase production capacity in the second half of this year.
Previously, Stacey Kennedy, President and CEO of PMI's US operations, announced during a press conference that PMI will be constructing a factory in Aurora, Colorado to produce ZYN nicotine pouches.
According to PMI, the $600 million investment in the factory is expected to bring 500 jobs to Colorado over the next two years. The factory is expected to hold a groundbreaking ceremony later this year and begin production in 2026.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








