Important Disclaimer Below — Please Read Carefully
2Firsts, September 6, 2025 —— Chemical Research in Toxicology (American Chemical Society) published “Heating Coil Corrosion by E-Liquid Containing Nicotine Lactate Salt and In Vitro and In Vivo Evaluation of Nickel Leachate in E-Liquid and E-Cigarette Aerosol.” Authors are from IMiracle (Shenzhen) Innovation Technology Co., Ltd., Dongguan Hongfu Biological Technology Co., Ltd., and Sun Yat-sen University. The work examines material compatibility between nicotine lactate and heating elements and the toxicological signals associated with Ni leachates.
Main Part
Methods
Disposable e-cigarettes with cotton-wicked atomizers and Ni–Cr coils (~80% Ni/20% Cr) were operated on a smoking machine (55 mL, 3 s, 2 puffs/min). Metals in e-liquids/aerosols/tissues were measured by ICP-MS; carbonyls by HPLC-DNPH; coil morphology by SEM/EDS. Aerosols were collected via cold trap for chemistry and cell exposures. Sub-chronic inhalation used male C57BL/6J mice in whole-body chambers (target 1000 mg/m³).
Main findings
• Time-dependent Ni leaching in e-liquid: When Ni–Cr coils were soaked in a model e-liquid (PG:VG 6:4) containing 2% nicotine lactate at 60 °C, Ni concentrations rose significantly after 1 week and continued to increase through 2 and 4 weeks. Under the same conditions, the 2% nicotine benzoate group did not show a comparable Ni rise; other metals (Al, Cr, Fe, Cu, As, Cd, Sn, Sb, Hg, Pb) remained largely unchanged. Both salt formulations were mildly acidic (pH ≈ 4.7–4.8).
• Elevated Ni in aerosol and puff-number effect: Using a commercial disposable device (liquid capacity 9.4 mL; 11 W) with Ni–Cr coils, aerosols from 2% nicotine lactate showed significantly higher Ni than aerosols from 2% nicotine benzoate within the first 100 puffs, with lactate-group Ni increasing further across 0–100, 200–300, and 400–500 puffs. Carbonyls (formaldehyde, acetaldehyde, acrolein, crotonaldehyde) were comparable between groups. Switching to Fe–Cr–Al coils reduced Ni to trace levels regardless of nicotine salt. SEM after 500 puffs revealed pronounced surface degradation and pitting on Ni–Cr coils with nicotine lactate, versus minimal damage with nicotine benzoate.
• In vitro and in vivo accumulation/toxicity: With matched nicotine content, cold-trap aerosols from the lactate formulation produced higher intracellular Ni accumulation and greater cytotoxicity in Beas-2B, SH-SY5Y, and HepG2 cell lines than aerosols from the benzoate formulation. In a 3-month whole-body inhalation study (C57BL/6J mice; 6 h/day, 5 days/week), Ni accumulated predominantly in the liver, and also in brain tissue, with significantly higher levels in the nicotine lactate aerosol group than in the benzoate group.
• Proposed mechanism: Nicotine lactate e-liquids displayed higher electrical conductivity than nicotine benzoate, suggesting greater ionic activity and electrochemical corrosion potential. At neutral pH (7), Ni release was similar between salts; at mildly acidic pH (5), Ni release rose sharply with nicotine lactate but not with nicotine benzoate—consistent with acid-accelerated metal dissolution. The authors conclude that both electrochemical and acid-mediated processes contribute to increased Ni release with nicotine lactate.
Authors’ conclusions
Under the tested conditions, 2% nicotine lactate e-liquids promoted corrosion of Ni–Cr heating coils and increased Ni transfer into e-liquids and aerosols, with corresponding in vitro and in vivo signals of higher Ni accumulation and toxicity. Coil material choice (e.g., Fe–Cr–Al) substantially influenced Ni release.
Limitations (as noted by the authors)
This is preclinical work (cell and animal models) conducted under specific formulations, temperatures, and puffing regimens; real-world devices and use patterns may differ. Water content and longer-term environmental variables of the PG/VG base were not comprehensively analyzed.
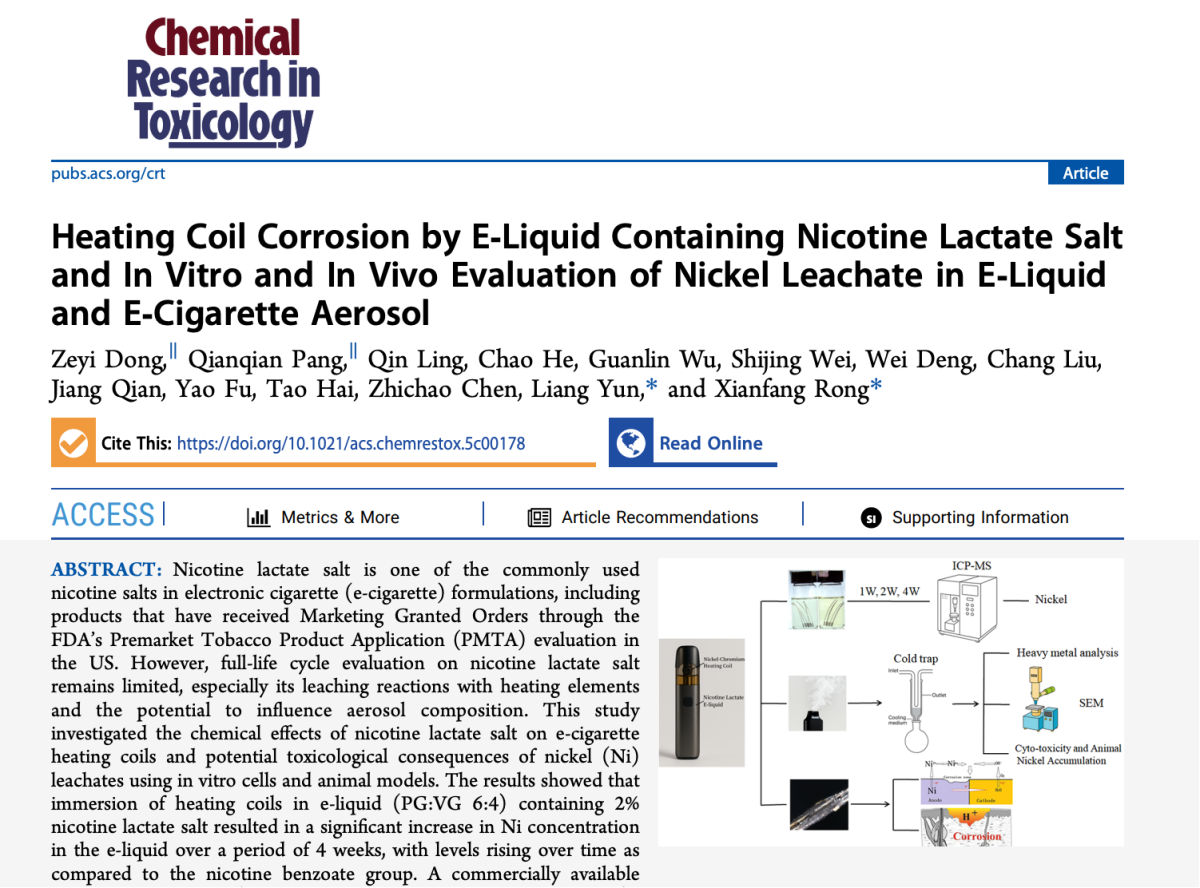
Title: Heating Coil Corrosion by E-Liquid Containing Nicotine Lactate Salt and In Vitro and In Vivo Evaluation of Nickel Leachate in E-Liquid and E-Cigarette Aerosol
Authors: Zeyi Dong; Qianqian Pang; Qin Ling; Chao He; Guanlin Wu; Shijing Wei; Wei Deng; Chang Liu; Jiang Qian; Yao Fu; Tao Hai; Zhichao Chen; Liang Yun*; Xianfang Rong* (*corresponding authors)
Publication Date: Sep 4 2025 (Accepted: August 27, 2025)
Journal: Chemical Research in Toxicology (American Chemical Society)
Cover image generated by ChatGPT
Disclaimer:
1.This article is a summary created by 2Firsts based on a published scientific paper. Its purpose is to make complex research findings more accessible to non-specialist audiences—particularly industry professionals, policymakers, and the media—in order to foster deeper connections between science, regulation, and the NGP industry.
2.Unless otherwise stated, the methods, findings, and conclusions presented in this summary reflect the views of the original paper’s authors. 2Firsts does not endorse any specific position and provides this content solely for informational dissemination.
3.Due to the limitations of our editorial and scientific capacity, there may be inaccuracies or misinterpretations in our summary. We welcome reader feedback and strongly encourage those interested to consult the original paper for a more accurate and comprehensive understanding.
4. 2Firsts supports open discussion and critical thinking around research. While no single study can answer all questions, we believe that open, rational dialogue helps us better understand the world and contributes to the sustainable development of tobacco harm reduction—ultimately enabling consumers to make healthier choices.
For feedback or collaboration, please contact us: info@2firsts.com








