
On the evening of June 2nd, 2FIRSTS received the latest response from the Medicines and Healthcare products Regulatory Agency (MHRA) regarding the registration status of "VEEBA," a disposable product by Philip Morris International (PMI).
Maria, the liaison officer from the MHRA News Center, confirmed that certain disposable VEEBA electronic cigarettes have been published by the MHRA, allowing them to be legally sold in the UK market. Their team is actively working to resolve an unexpected technical error on the public-facing page to ensure the visibility of all published products.
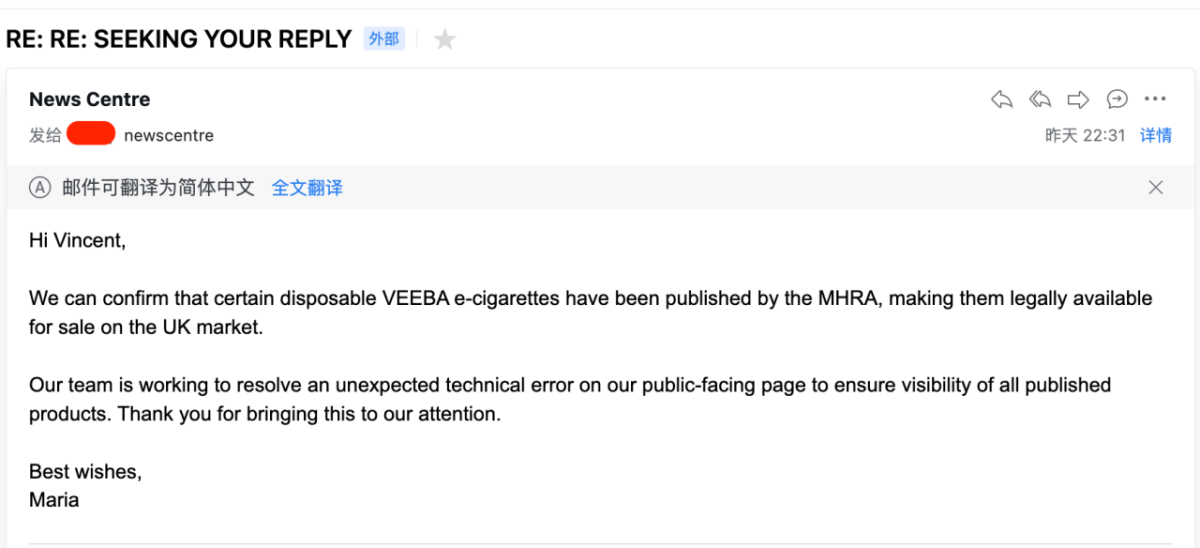
Maria concluded the response by expressing thanks on behalf of the MHRA to 2FIRSTS and reminding them that the agency is aware of the issue.
As of the time of publication, 2FIRSTS' search on the MHRA platform still shows no results for VEEBA electronic cigarettes.
2FIRSTS previously sent inquiry emails to PMI on May 29th. However, PMI has not yet responded to this matter.
This document has been generated through artificial intelligence translation and is provided solely for the purposes of industry discourse and learning. Please note that the intellectual property rights of the content belong to the original media source or author. Owing to certain limitations in the translation process, there may be discrepancies between the translated text and the original content. We recommend referring to the original source for complete accuracy. In case of any inaccuracies, we invite you to reach out to us with corrections. If you believe any content has infringed upon your rights, please contact us immediately for its removal.


