Juul is no more, but the brand only accounts for a fraction of Colorado’s teen vaping.
The U.S. Food and Drug Administration banned the sale of Juul products on Thursday and ordered the company to pull its products from store shelves. The action is part of a larger effort to curb teen vaping rates.
FDA bans Juul e-cigarettes tied to teen vaping surge
This should hit Colorado’s vapers hard. The state has the largest rate of teen vape users, according to 2017 survey data from the U.S. Centers for Disease Control and Prevention. This follows one of the nation’s highest overall vaping rates. Colorado ranked fourth in the nation in 2018, with 7.3% of its population using vape products.
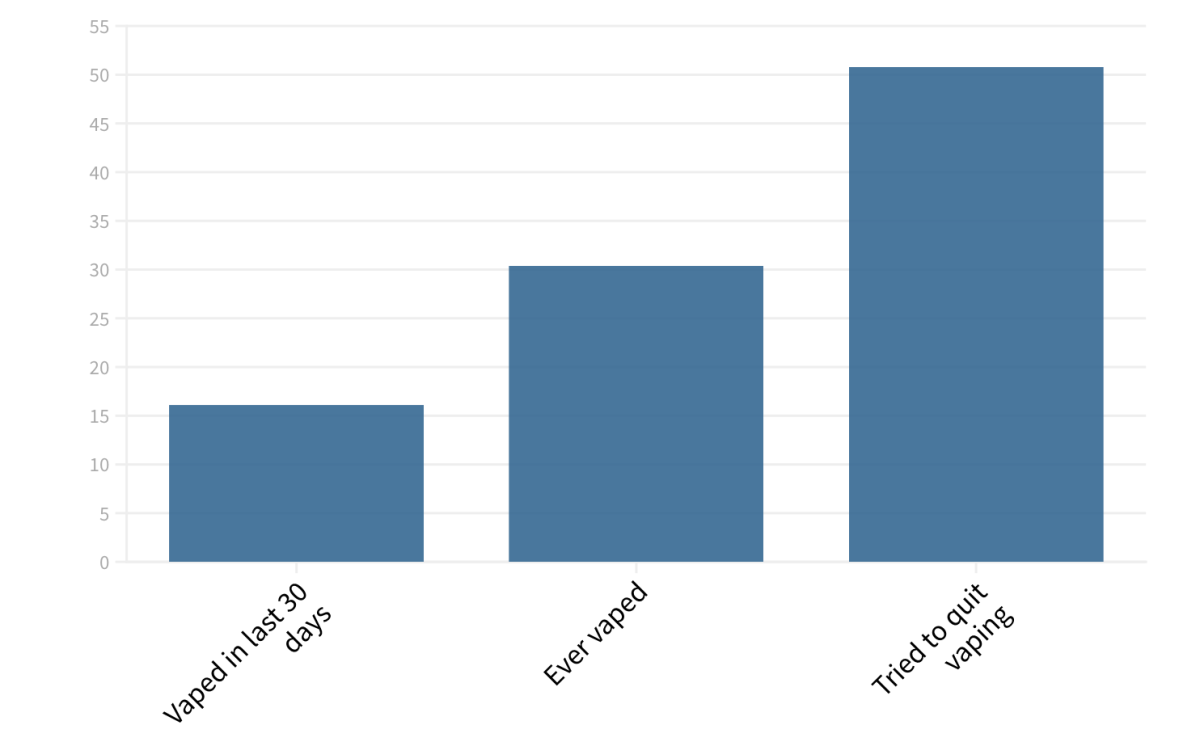
High schoolers vape at even higher rates, according to the state health department’s Health Kids Colorado Survey.
Among Colorado high schoolers, 30.4% have vaped at least once. Just over 16% have vaped in the last 30 days. Users aren’t simply dabbling, either. Half of the high schoolers who vaped in the last 30 days were trying to quit.
Juul is not the preferred product, however.
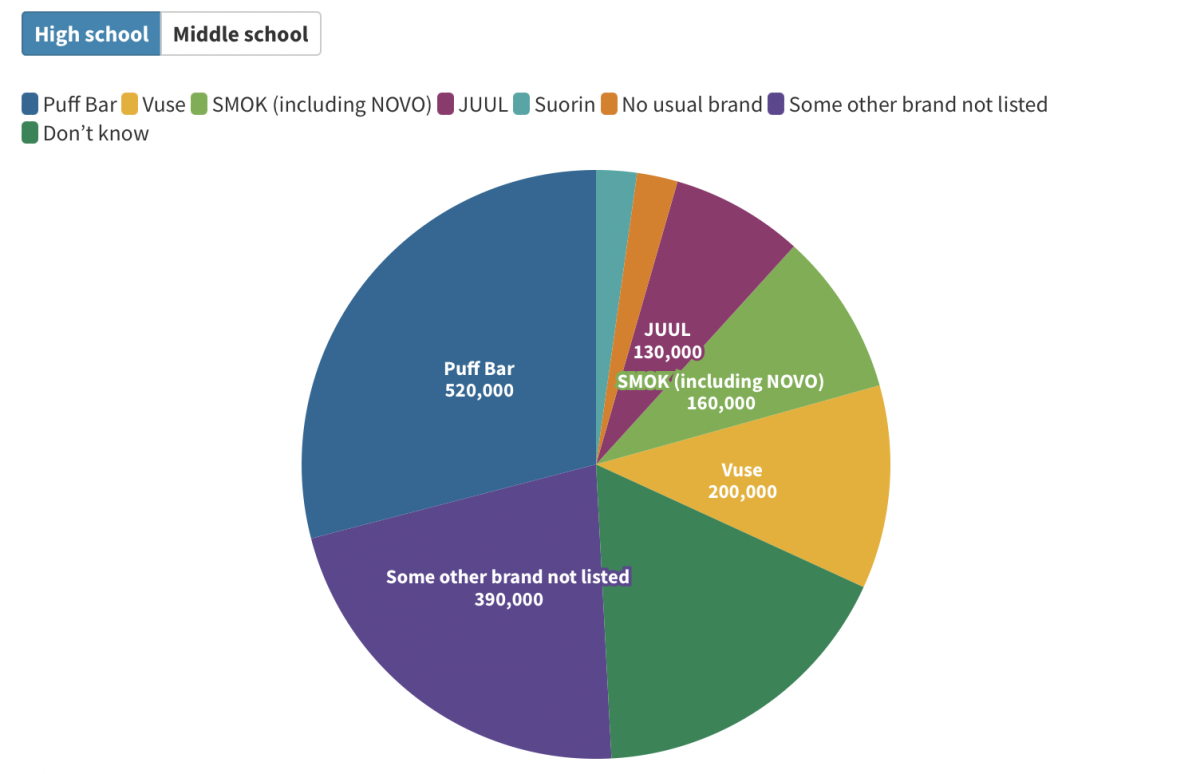

Nationally, 130,000 high school vape users prefer Juul, according to a CDC study. This only constitutes about 7%. Among middle schooler vape users, only 6% prefer Juul.
Puff Bar, Vuse and SMOK are more popular brands, though roughly one in five high school vape users admits to not knowing the brand they consume.








