Editor's Note:
After the publication of this article, some experts pointed out that Import Alert 98-06 is aimed at products rather than companies, that is, this time it targets the RAZ DC25000 ENDS products, not all products of Qisitech.
For the sake of accuracy, this article has been specially updated.
Recently, the official website of the U.S. Food and Drug Administration (FDA) updated Import Alert 98-06. 2Firsts noticed that a product of Zhuhai Geekvape Technology Co., Ltd. (hereinafter referred to as "Geekvape"), was included in the latest import alert list.

According to the FDA website, ZHUHAI QISITECH CO., LTD was listed in the "Import Alert 98-06" also known as the "Red List" updated on September 26th, titled "Detention Without Physical Examination of New Tobacco Products Without Required Marketing Authorization".
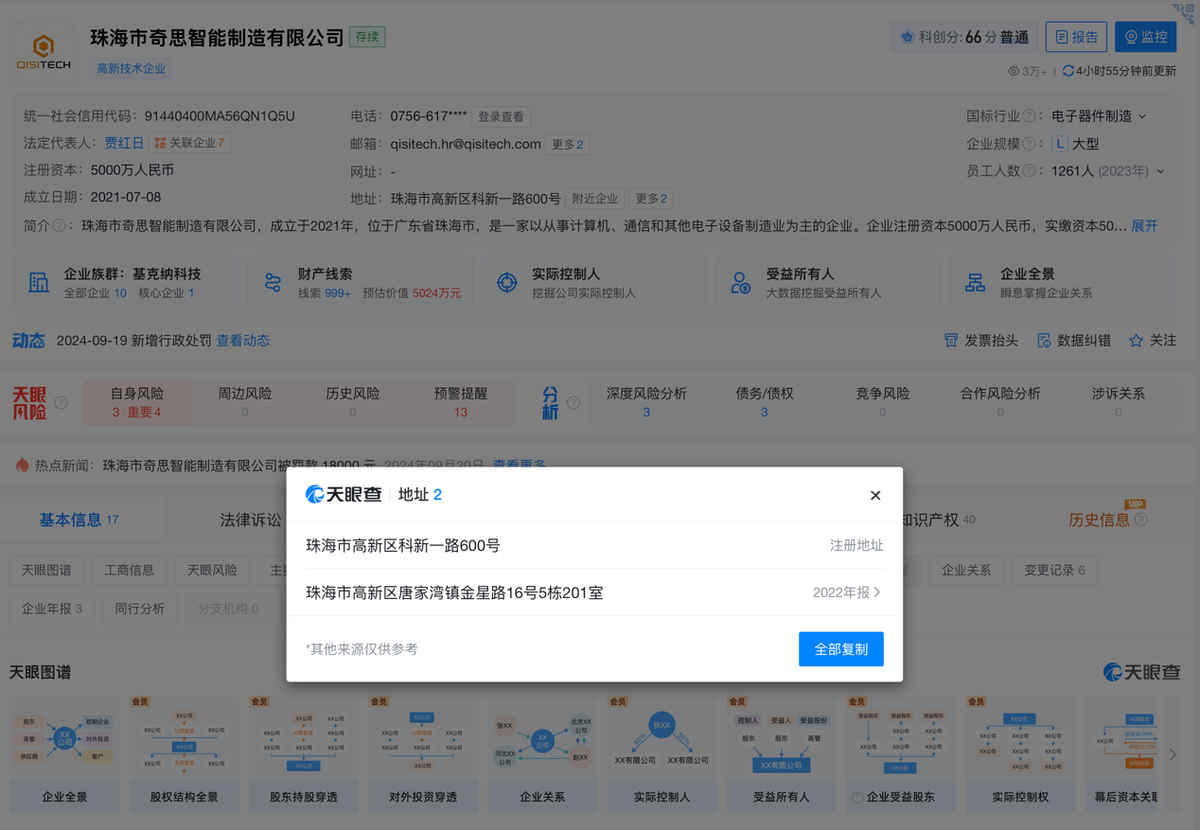
Comparison between information released by the FDA and information from Tianyancha shows that ZHUHAI QISITECH CO., LTD, also known as Zhuhai Qisi Manufacturing Co., Ltd., was established in 2021 and is a wholly-owned subsidiary of Shenzhen Geekvape Technology Co., Ltd. Geekvape is the brand owner of Geek Bar and Geek Vape, two of the most well-known e-cigarette brands in the United States, while Qisitech is one of the main contract manufacturers under Geekvape.
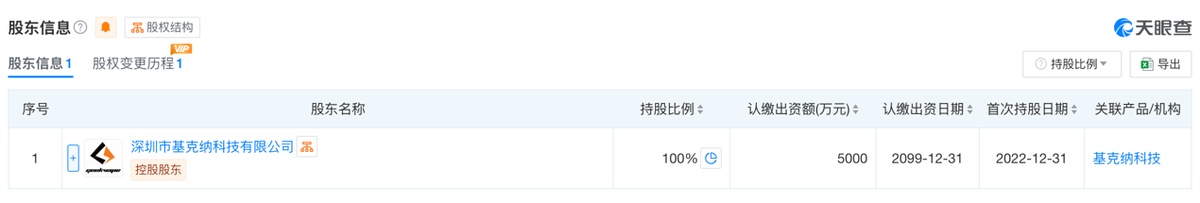
According to the FDA official website, departments can detain tobacco products listed on the Red List of this Import Alert without needing to conduct physical examinations. If a company wants to remove their products from the list, they must provide information to the relevant agencies to demonstrate that they have addressed the issues leading to non-compliance.
According to the above provisions, Qisitech's exports of the mentioned product to the United States will be detained until they are removed from Import Alert 98-06 list.
Compliance expert Kurt informed 2Firsts that Import Alert 98-06 is a list of companies that have submitted PMTAs (Pre-Market Tobacco Product Applications). Meanwhile, Import Alert 98-07, as listed on the FDA website, is focused on companies that have submitted PMTA applications.
2Firsts will continue to monitor the development of the event and provide timely updates on related coverage.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








