After pausing its ban of the popular Juul e-cigarettes, the U.S. Food and Drug Administration faces mounting pressure to crack down on another type of vaping product favored by teens and young adults.
Anti-tobacco groups and lawmakers have raised questions over the agency's efforts to rein in synthetic nicotine products, often disposable and sold in flavors favored by underage vapers. The focus on this increasingly popular form of vaping comes after the FDA halted its ban on Juul this month for additional review.
"FDA has continually been too slow to act and too tentative in its actions," said Matthew L. Myers, president of the Campaign for Tobacco-Free Kids, a nonprofit that works to reduce tobacco use. "The failure of FDA to act decisively is the reason that the youth e-cigarette epidemic grew out of control and continues today."
In an effort to skirt FDA regulations, experts say, vaping companies began selling nicotine made in a lab rather than from tobacco. A law passed by Congress gave makers of synthetic nicotine products until May 14 to apply to market these products. Companies that failed to get authorization by July 13 are selling the products illegally, according to the FDA.
FDA Commissioner Robert Califf said in a Twitter post that all non-authorized products are illegal and subject to enforcement. The FDA warned two manufacturers last week that they were illegally marketing such products. Letters also went out to another 107 retailers warning about unlawfully selling the products.
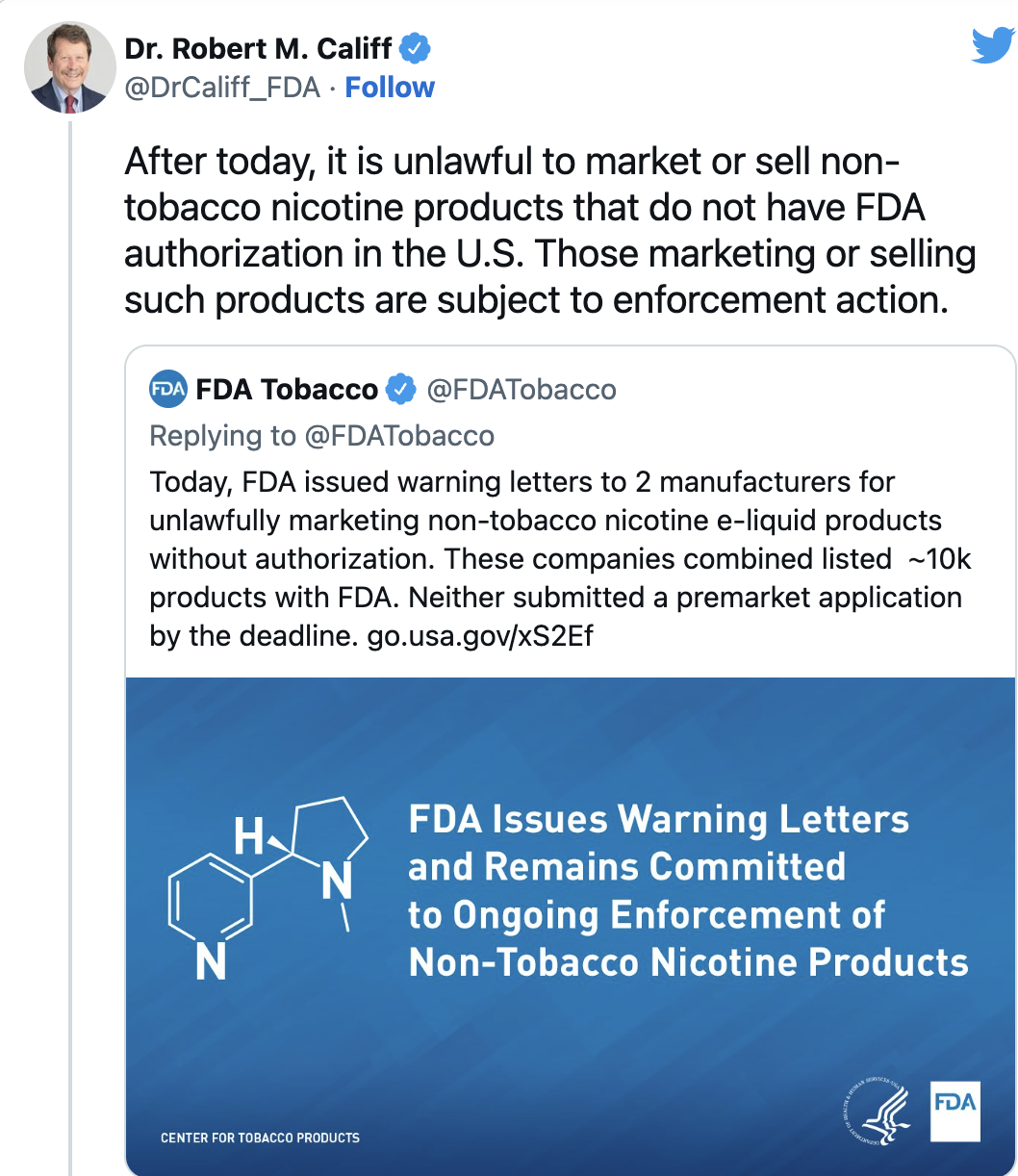
But the FDA has not yet removed most products despite calls to do so from anti-tobacco groups and some lawmakers. Robin Koval, CEO and president of Truth Initiative, a public health organization funded by a 1998 settlement between states and the tobacco industry, called on the agency to "immediately remove all synthetic nicotine products" from the market.
U.S. Sens. Dick Durbin, D-Ill., and Susan Collins, R-Maine, said in a letter last week that the agency's failure to vet and remove non-authorized products could have "grave consequences for the health of children across America."
The FDA said it has received applications for about 1 million synthetic nicotine products and will soon issue "refuse to accept" letters for those that don't past muster. The agency typically sends letters to get companies to comply and follows with fines, formal orders, seizures or court injunctions.
Puff Bar, flavored vapes popular with teens
Anti-tobacco groups say companies marketing synthetic nicotine products are enticing young vapers with flavored products. Puff Bar, which markets flavors such as banana ice, blue razz and grape, is among the most popular products among young vapers. Last year, 30% of middle school and 26% of high school e-cigarette users named Puff Bar their usual brand, according to the 2021 National Youth Tobacco Survey.
Puff Bar CEO Patrick Beltran defended his company.
"We have never sold to underage users," Beltran told USA TODAY. "A lot of the responsibility obviously falls on companies being responsible, but also on retailers and the government going after people that are bad players and selling to underage users."
Beltran added his company continues to market its products to retailers and submitted a "very thorough" application by the deadline. He said the company has not heard from the FDA about its application.
Because the company is trying to comply with the FDA's rules, he said, "there's no loophole in regards to this synthetic nicotine. We believe synthetic nicotine is better than tobacco-derived nicotine."
The two companies warned last week by the FDA each marketed flavored synthetic nicotine products. AZ Swagg Sauce LLC listed for sale blue samurai, butter cracker and berry cheesecake vape juice flavors. Electric Smoke Vapor House sold churro, cookie dough and orange Creamsicle products, the FDA said. The companies have 15 days to respond to the FDA's warning letter. AZ Swagg Sauce did not immediately return e-mails or calls.
Kimberly Hammond, who owns Electric Smoke Vapor House in Tullahoma, Tennessee, said the application was too onerous to complete for her single-shop vape house. The agency rejected her application in 2020, she said, and she didn't have the resources to go through the process again.
She said "labs are few and cost is high" to test each flavor and nicotine level for all her products.
"Between the expense, time and stress, it's quite overwhelming," Hammond said. "And in the middle of it, I threw up my hands."
Myers said vaping companies marketing synthetic nicotine products are doing so to get around the FDA's ban on flavored products derived from tobacco.
"Increasingly, kids turn to the synthetic products because they come in flavors that have otherwise been banned," Myers said. "Flavors hook kids, (and) the industry used synthetic products to avoid FDA regulation. Congress gave FDA both the authority and the mandate to act decisively and, at least today, FDA has not done so."
Koval said the FDA is in the "impossible situation" of trying to review millions of applications to sell both traditional tobacco-derived nicotine and synthetic nicotine products. In 2020, the FDA required all e-cigarette and vaping companies to submit applications to continue marketing products and banned fruit- and mint-flavored juice pods in nicotine derived from tobacco.
She urged the agency to prioritize reviews of e-cigarette companies that sell the most products and are popular among kids. She added the FDA should remove from the market all non-authorized e-cigarettes, even those whose manufacturers have submitted applications for which the agency has not completed reviews.
"Time is of the essence as every day of delay results in hundreds of new products flooding the market, more young people taking their first hit and smokers uncertain whether these products will truly help or are even safe to use," Koval said in a statement.
The case of Juul
Last month, the FDA banned the sale and marketing of Juul vaping and e-cigarette products because of what the agency described as insufficient and conflicting data in the company's application to stay on the market. The San Francisco-based vaping company sued and won a temporary order to stay on the market.
On July 5, the FDA said it would suspend its Juul ban, citing "scientific issues unique to the Juul application that warrant additional review." The agency did not describe what those issues included.
In a joint filing to the U.S. Court of Appeals for the District of Columbia, the FDA agreed to pause its Juul ban while it conducts a review. Even if the agency decides to maintain the ban, it will give Juul 30 days to seek court relief before enacting the ban.
Peter Pitts, a former FDA associate commissioner and co-founder of the Center for Medicine in the Public Interest, a nonprofit research and educational organization, said the FDA's backtracking does not reflect well on its attempt to rein in the biggest name in the vaping industry.
"It puts a stain on the FDA's credibility relative to making impartial, nonpolitical, science-based decisions," Pitts said.
The content excerpted or reproduced in this article comes from a third-party, and the copyright belongs to the original media and author. If any infringement is found, please contact us to delete it. Any entity or individual wishing to forward the information, please contact the author and refrain from forwarding directly from here.







