
Key Takeaways:
· Launch Date: Official U.S. release on October 14, 2025.
· Initial Markets: North Carolina, Texas, and Florida.
· Product Features: Spit-free design featuring NICOSILK™ mesh technology.
· Regulatory Status: PMTA submission to the FDA exceeds 25,000 pages.
· Market Context: ZYN remains the only FDA-authorized brand; competition is heating up.
2Firsts, October 14, 2025 — Altria’s nicotine pouch brand On! is set to officially enter the U.S. market. The new product, On! PLUS™, developed by Altria subsidiary Helix, will first roll out in North Carolina, Texas, and Florida.
The news first surfaced in the Reddit nicotine pouch community (r/NicotinePouch), where multiple users said they had received launch notification emails from On!. According to shared posts, initial flavors include Mint, Wintergreen, and Tobacco, with some users noting that onnicotine.com may offer mail-order purchases.
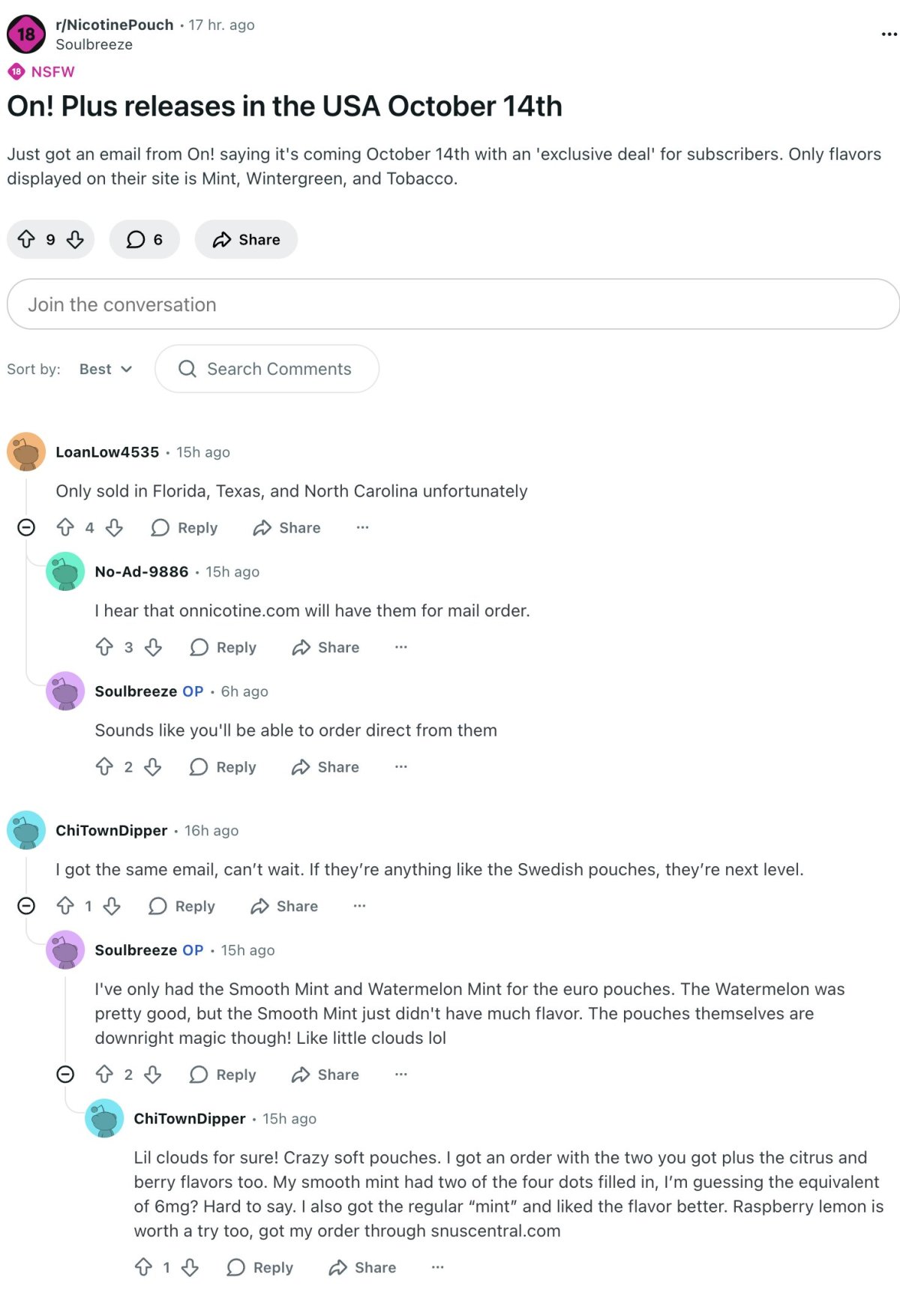
Consumers generally expressed enthusiasm for the new product and compared it to On! offerings in Europe. Some users described the European On! pouches as “soft-bodied with a clean taste,” praising flavors like Smooth Mint and Watermelon Mint.
Helix moves first on market deployment
As early as August 22, 2Firsts observed that Altria announced via its official account on X (formerly Twitter) that Helix would launch On! PLUS™ this fall. Made at the company’s Richmond, Virginia facility, the product targets adult consumers 21+, offers 6 mg, 9 mg, and 12 mg nicotine strengths, and features the proprietary NICOSILK™ mesh technology to provide U.S. consumers a more convenient smoke-free alternative.
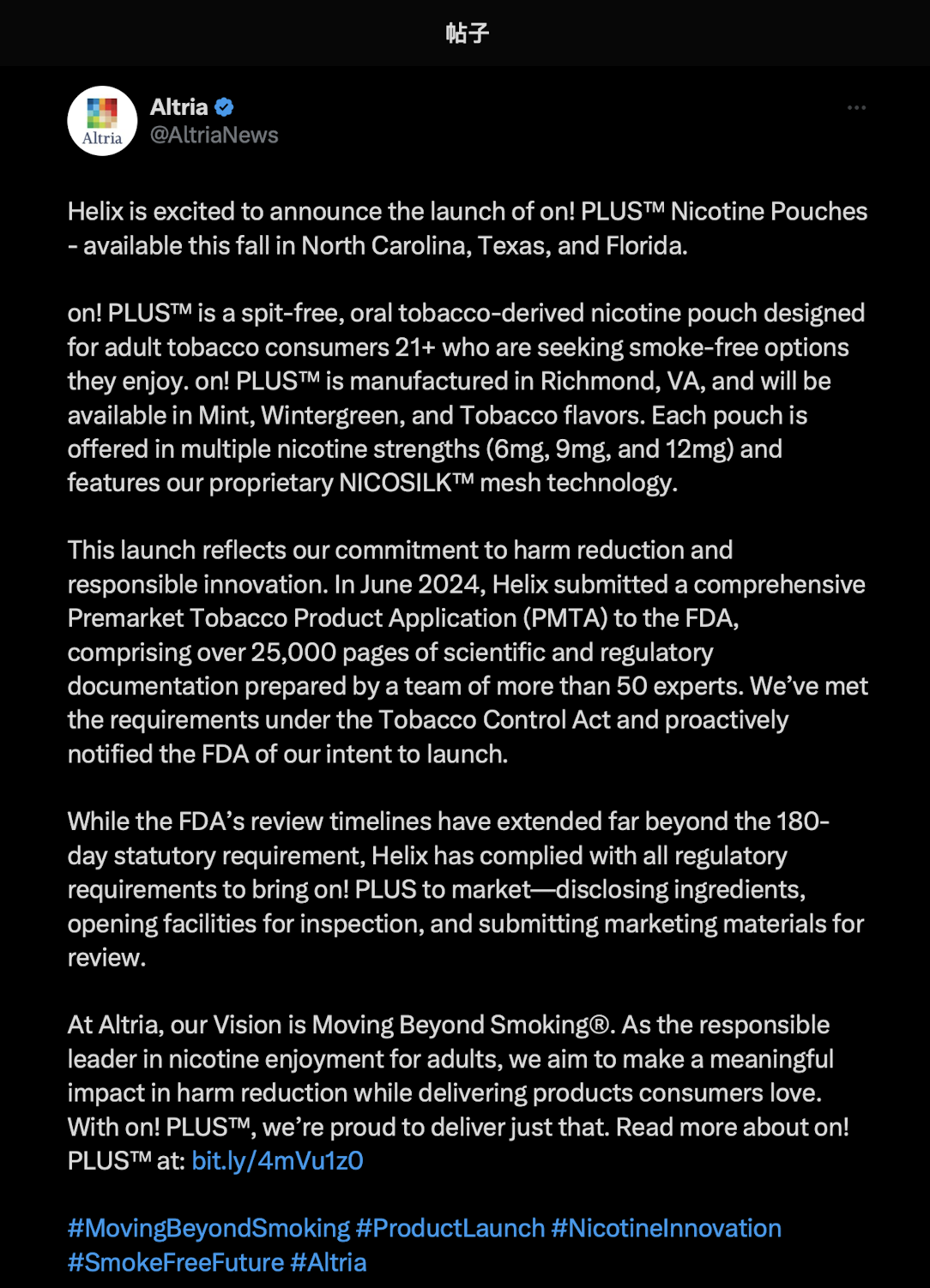
Although FDA authorization has not yet been granted, Altria plans to proceed in accordance with compliance procedures. Helix submitted a PMTA in June 2024 exceeding 25,000 pages, authored by more than 50 experts and covering scientific and compliance studies in line with the Tobacco Control Act.
Altria stated that while the FDA review has surpassed the statutory 180-day timeframe, the company continues to follow all compliance steps, including ingredient disclosures, facility inspections, and marketing material submissions.

Industry backdrop: competition in smoke-free alternatives intensifies
At present, ZYN—owned by Philip Morris International (PMI)—is the only nicotine pouch brand authorized by the FDA for U.S. sales. No other brand has received full marketing authorization.
Notably, just one day before Altria announced On! PLUS™ (on August 21), Reuters reported that British American Tobacco (BAT) plans a U.S. pilot for its disposable synthetic-nicotine e-cigarette Vuse One, expected to launch in late September in South Carolina, Florida, and Georgia. The overlapping timelines and regions with On! PLUS™ suggest a new wave of competition in the U.S. nicotine alternatives market.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








