![[Breaking] Juul’s Device and 4 Pod Products Officially Authorized by FDA, Reenters the U.S. Regulated Market](https://static.2firsts.com/uploads/20241113/34ffc17feb2b6076367c9e5f413641f2.jpg?x-oss-process=style/origin)
Reporter | 2Firsts
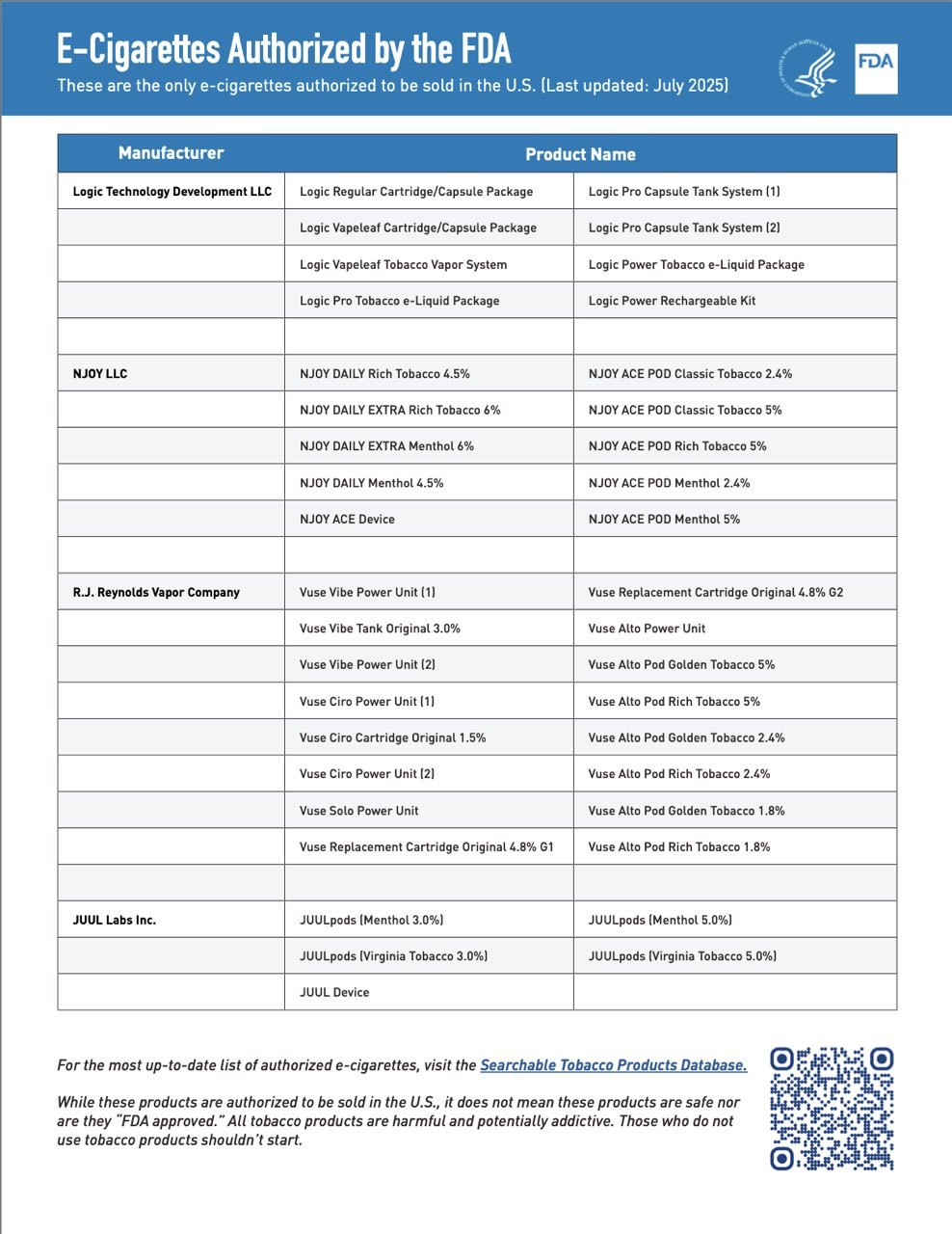
According to the latest update of the FDA’s List of Authorized E-Cigarette Products (July 2025), Juul Labs Inc. has officially received Marketing Granted Orders (MGOs) for five of its products, including:
· JUUL Device
· JUULpods [Menthol 3.0%]
· JUULpods [Menthol 5.0%]
· JUULpods [Virginia Tobacco 3.0%]
· JUULpods [Virginia Tobacco 5.0%]
This marks Juul’s return to the FDA’s list of authorized e-cigarette products, three years after the agency issued a market ban in 2022. The approved products include Juul’s core menthol and Virginia tobacco flavored pods.
To date, the FDA has granted authorization to e-cigarette products from four manufacturers: Logic, NJOY, Reynolds (Vuse), and now Juul. The total number of authorized products has increased from 34 to 39.
In July 2024, RJ Reynolds Vapor Company, a subsidiary of British American Tobacco, secured FDA authorization for seven Vuse Alto products. Juul’s approval represents the first new MGO issued in over a year, signaling a potentially significant shift in the U.S. e-cigarette regulatory landscape.
2Firsts will continue to monitor Juul’s market reentry and its broader impact on the industry.








