
Special statement:
This article is for internal industry communication only and does not make any recommendations for specific brands or products.
The images presented in this article are only used to describe the facts and are not intended as advertisements for any products.
Minors are prohibited from accessing this article.
Product Highlights:
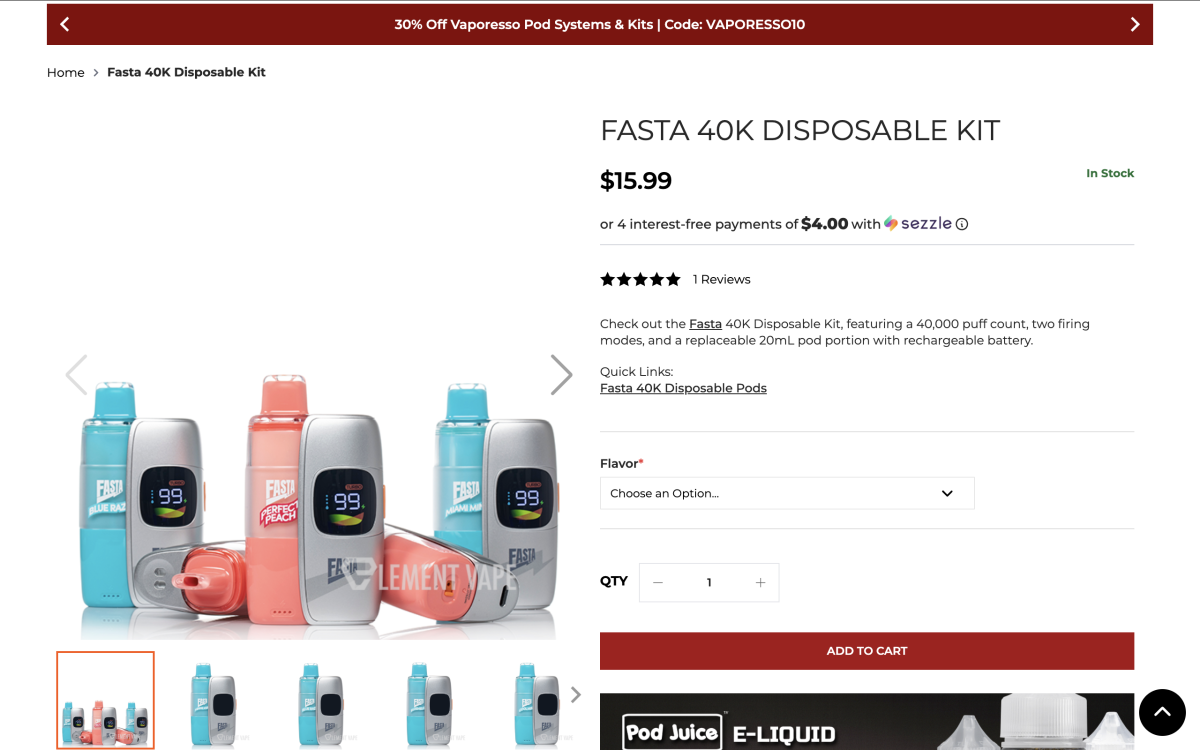
Design & Build: The FASTA 40K Kit uses a modular magnetic connection: a reusable “large battery base” paired with prefilled disposable pods. The device includes a small display showing battery level, e-liquid level, and mode.
Modes: Two power levels, plus two-stage airflow adjustment.
Endurance & Specs: Rated 20 mL e-liquid and 40,000 puffs. The pod side lists 200 mAh; combined with the base, ratings shown are 1350–1550 mAh (varies by listing). Nicotine is labeled 50 mg/mL (5%). This is a disposable-pod + reusable-battery format.
Market & Price: Available/on sale across multiple U.S. retailers; Element Vape lists it at $15.99.
2Firsts, October 14, 2025 — 2Firsts has observed that FASTA’s new vape, the FASTA 40K Kit, has gone live across several U.S. channels. The product adopts a magnetic, modular design with 20 mL prefilled pods + a reusable battery, is rated for 40,000 puffs, features a mini display, and supports Norm/Turbo modes with two-stage airflow. Channel listings show the kit around $15.99 and pods around $13.99 (subject to retailer).
Magnetic design, up to 40,000 puffs
2Firsts has compiled the key specs of the FASTA 40K Kit as follows (see chart):

According to multiple retailer pages, the FASTA 40K uses a prefilled pod (20 mL) + reusable battery base modular setup. Pods connect to the base magnetically; changing flavors requires swapping pods.
The device’s mini screen shows battery, e-liquid level, and power mode. It offers Norm/Turbo two-level output and two-stage airflow to balance flavor and battery life.
Listings also mention a dual-mesh coil to enhance flavor and vapor. The device is rated at 40,000 total puffs and 20 mL capacity—approximately 2,000 puffs per mL.
For batteries, the pod side provides 200 mAh auxiliary power; the large base is rated 1350 mAh.
“Disposable Kit” positioning; kit priced at $15.99
The FASTA 40K Kit is available on multiple U.S.-facing vape retail sites, including Element Vape / Vaping.com / Mi-Pod / VapeSourcing. Pod pricing is about $13.99, and the kit is $15.99. Mi-Pod shows several FASTA 40K Kit flavors as SOLD OUT.

On ELEMENT VAPE and VapeSourcing, the product is described as “Fasta 40K Disposable Kit.”
In the U.K. vape trade, similar magnetic designs are commonly referred to as “Prefilled Pod Kits.”
FASTA has recently launched multiple U.S. products
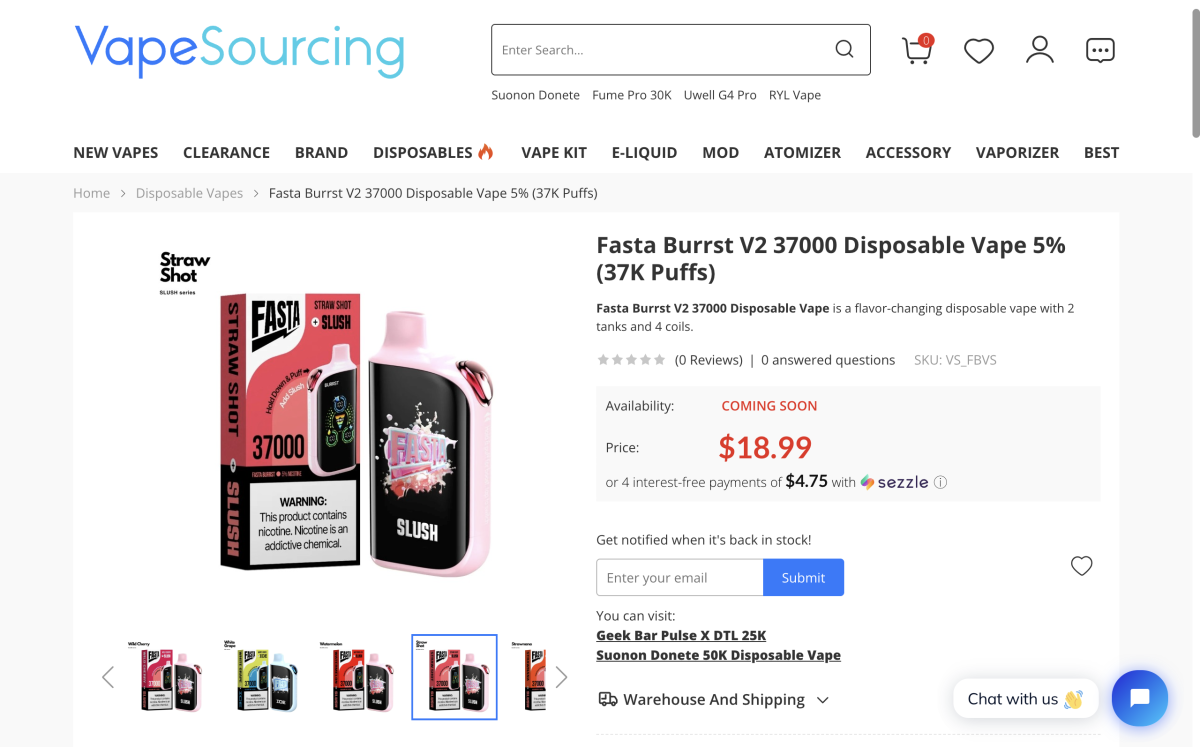
In addition, in September, FASTA released the Fasta Burrst V2 37000 in the U.S. As previously reported by 2Firsts, it retains a large display while increasing puff count to 37,000 and offers Ice / Sour / Creamy / Smoothie flavor tuning versions. It’s priced at $18.99 and listed on multiple U.S. retail sites.
As of this publication by 2Firsts, FASTA 40K Kit and Fasta Burrst V2 37000 have not received U.S. FDA market authorization under the PMTA process.
Cover image credit: tenvape
As a leading global NGP media and think tank, 2Firsts is dedicated to providing the latest product and technological information and insights to practitioners around the world in various categories such as e-cigarettes, heated tobacco products, and modern oral products. It aims to drive technological changes and innovations in NGP products worldwide, thereby offering tobacco consumers globally with safer products and lifestyles.
With a source of information covering the supply chains in China and global markets, 2Firsts product coverage has become one of the most influential platforms for new product and technology releases globally.
Contact 2Firsts for the following services:
1. Providing leads on new products and technologies;
2. Offering comments on products and technologies;
3. Seeking media coverage for your products;
4. Identifying sales channels for products.
Contact Information: Email: info@2firsts.com
Contact CEO Alan Zhao of 2Firsts on LinkedIn.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







