
The 2022 settlement agreement between e-cigarette company Juul and tobacco company Altria has begun to be paid out, with users on platforms such as Reddit and X claiming to have already received thousands of dollars in compensation, according to a 22 October report from Forbes.
The settlement amount for this case is $3 billion, to be paid jointly by Juul and Altria. The lawsuit alleged that consumers paid inflated prices for e-cigarettes, that Juul failed to accurately disclose the addictive and safety risks of its products, and that it engaged in illegal marketing to minors.
The payouts were approved earlier this month, according to the r/juul subreddit. Users have reported receiving amounts ranging from a few hundred dollars to over $9,000. Some X users have shared screenshots of their payments, while others are disappointed that they were not included in the settlement list. The settlement agreement includes consumers who purchased Juul products before 7 December 2022. Juul did not admit any wrongdoing in the settlement, and Altria has denied the allegations against it.
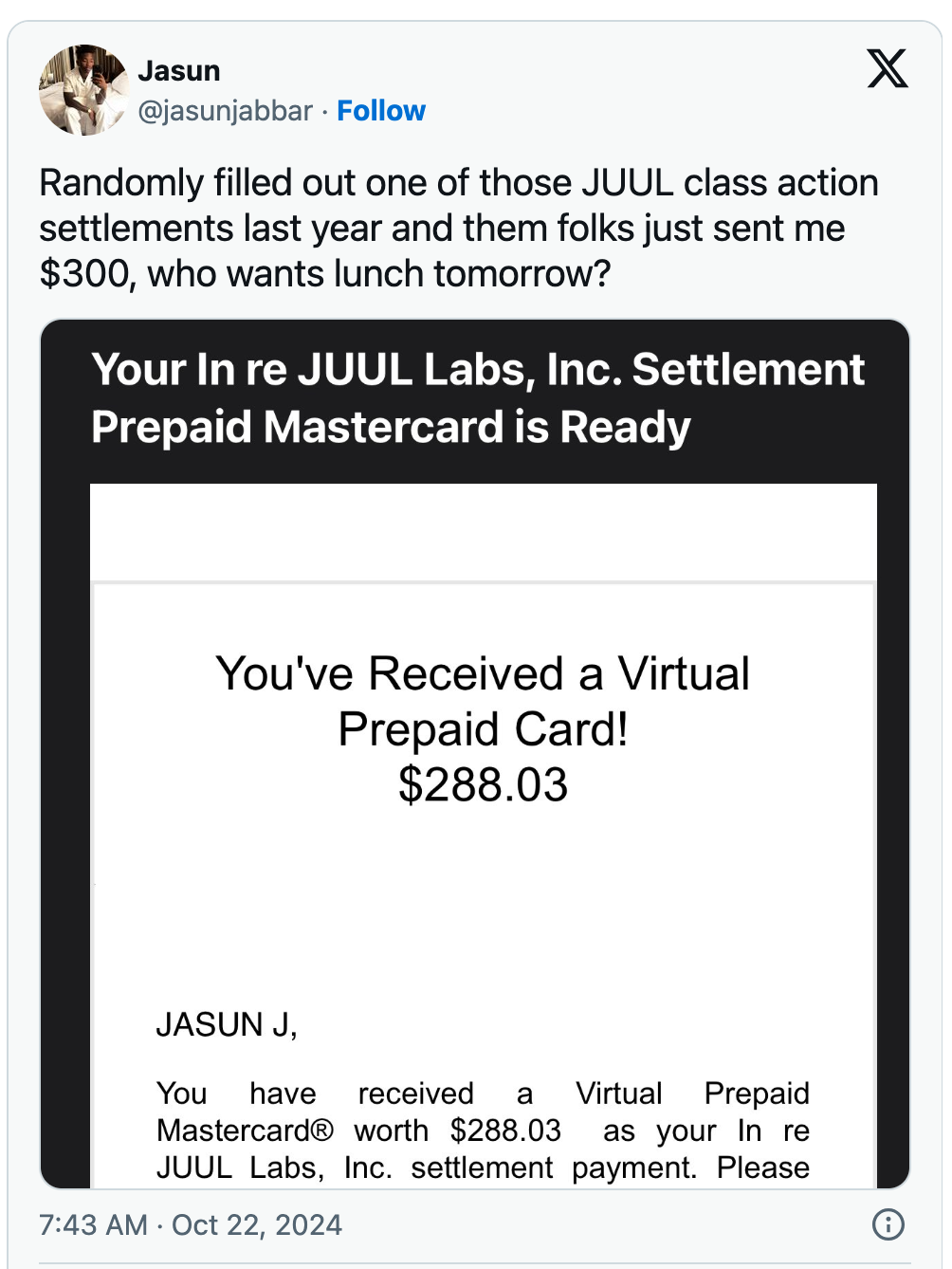
The amount of compensation is influenced by several factors, including the amount the claimant spent on Juul products, the time period of the purchase, and the age at the time of the purchase. Obviously, users who spent more during the qualifying period will receive more compensation.
The class action stems from more than 8,000 lawsuits filed against Juul by municipalities, school districts, indigenous tribes and individuals. In 2019, Juul stopped advertising in the US market and discontinued most of its flavoured products due to legal disputes and government sanctions.
Following a multi-state investigation into its marketing practices, the company reached a settlement agreement for nearly $4.4 billion, followed by another agreement for $3 billion. In 2018, Altria had invested nearly $13 billion in Juul, but pulled out of the investment last year and invested in another competing startup. Juul avoided bankruptcy through cost-cutting, massive layoffs and support from wealthy investors.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








