
According to a report by CNA Lifestyle on September 18th, Baekhyun, a member of the famous South Korean boy group EXO, sparked controversy after being photographed smoking in a restaurant in Macau, China.
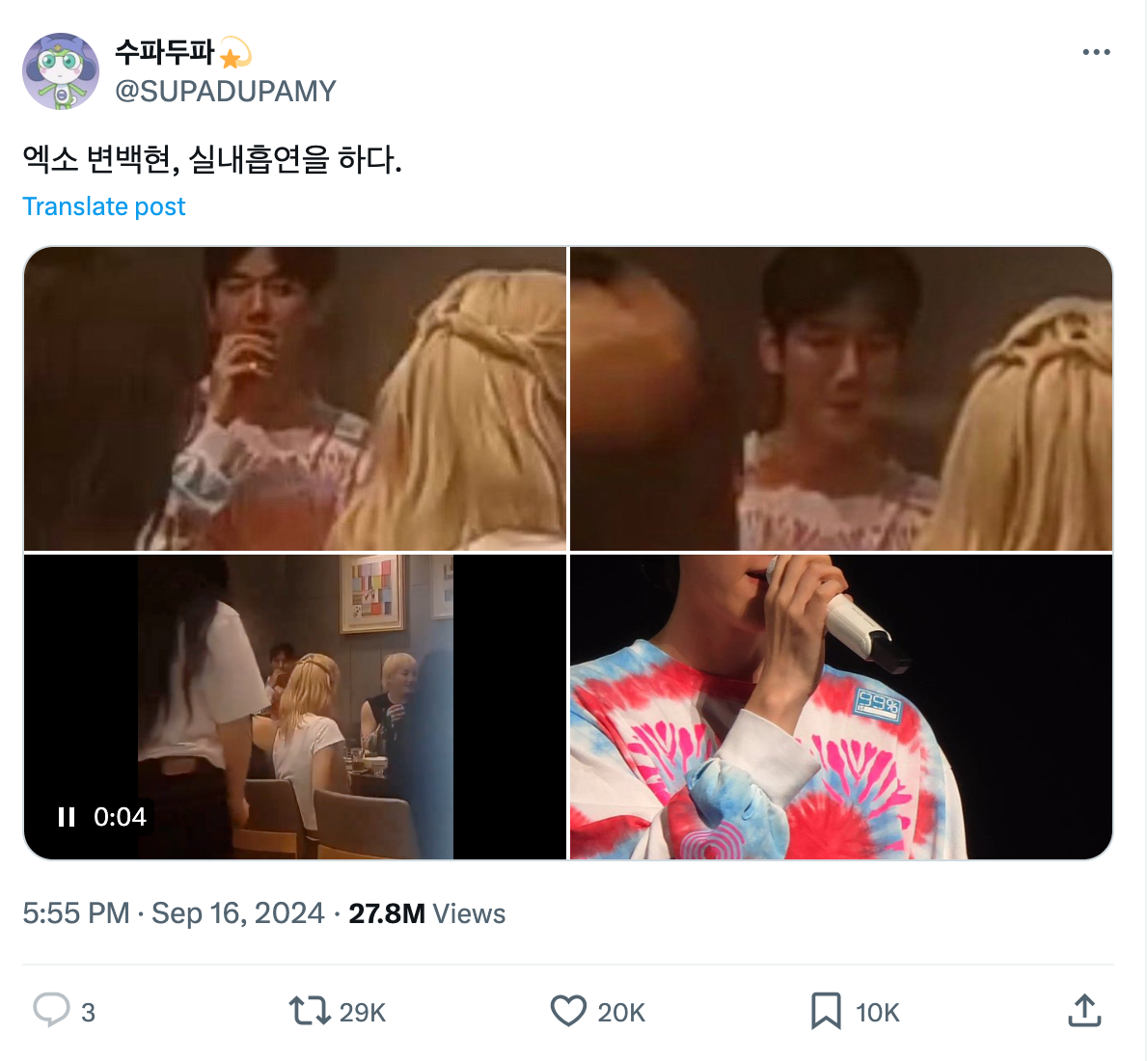
On September 16th, a video was exposed on X showing EXO's Baekhyun smoking an e-cigarette inside a restaurant in Macau, China. In the video, Baekhyun was seen smoking in a corner while chatting with several individuals who appeared to be staff members.
According to the laws of Macau, individuals are not allowed to bring e-cigarettes into the territory and smoking is prohibited in all indoor areas except for designated smoking rooms.
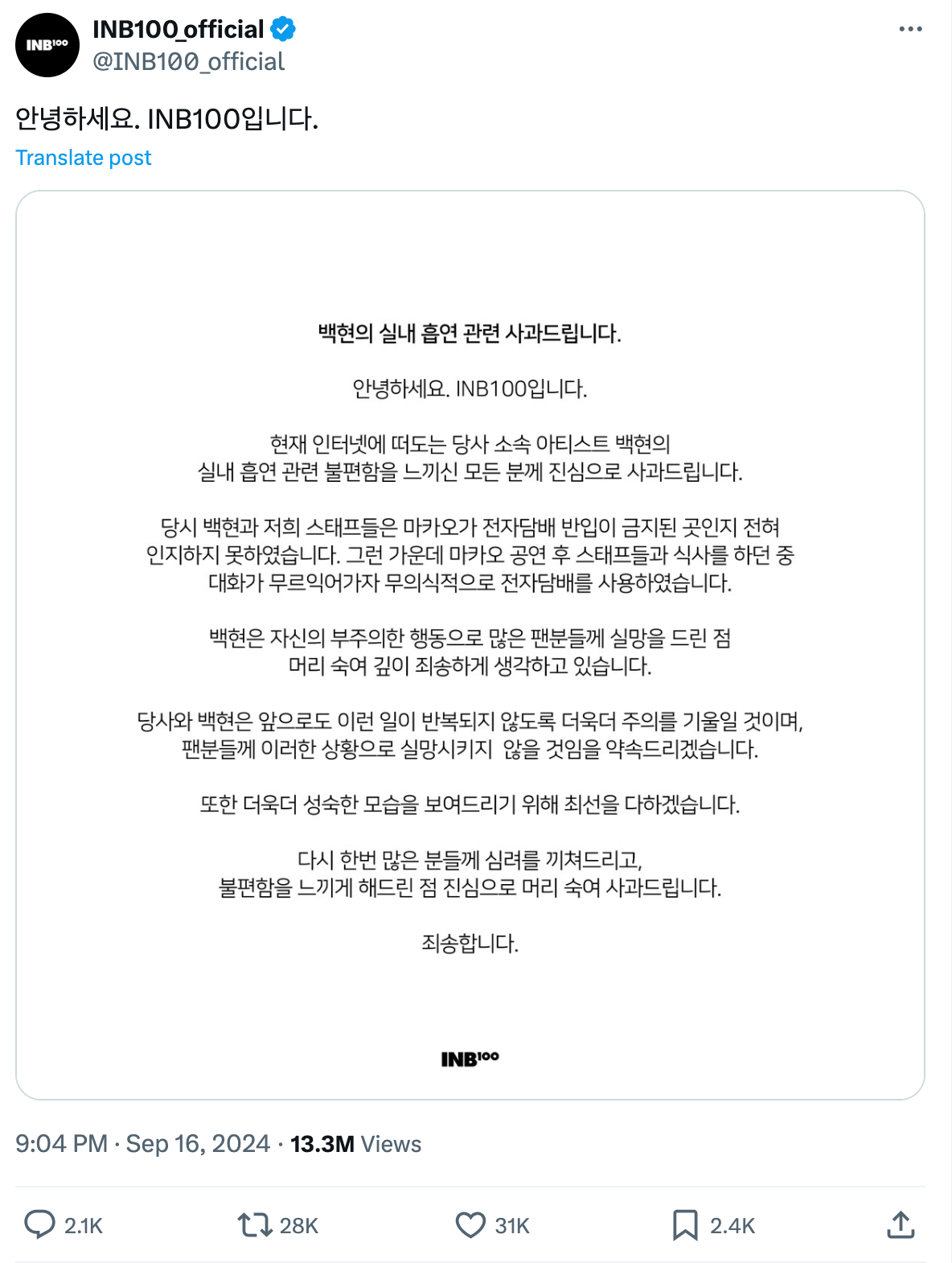
On September 16th, the management company INB100, representing Byun Baekhyun, issued a public apology statement through its social media. The company explained that the video was filmed after his concert in Macau in June this year, and that neither he nor the staff were aware of the prohibition of e-cigarettes in Macau, China.
The statement reads as follows:
Hello, I am INB100.
We apologize to everyone who felt uncomfortable about the recent controversy involving our artist, Baekhyun, smoking indoors.
At the time, Baekhyun and our staff were completely unaware that smoking e-cigarettes was prohibited in Macau. After Baekhyun and the staff finished their performance in Macau, they went out for dinner. As the conversation unfolded, Baekhyun unintentionally smoked an e-cigarette.
Due to his careless actions, Baekhyun deeply apologizes for disappointing many fans.
Baekhyun and our company will be more vigilant to ensure that such incidents do not happen again, and pledge not to disappoint fans in the future. We will also strive to behave in a more mature manner.
We once again express our sincere apologies for any concerns and discomfort we may have caused you.
We are truly sorry.
A few months ago, Blackpink member Jennie faced criticism after being photographed using an e-cigarette indoors.
According to reports, Byun Baekhyun debuted as a member of the South Korean boy group EXO in 2012 and has been very popular since his debut. EXO is divided into two sub-units, EXO-K and EXO-M, which promote in South Korea and China respectively. Wu Yifan (Kris), Lu Han, and Huang Zitao (Tao) were former members of EXO-M but left the group later due to personal reasons.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







