
According to a report on the official website of the US Food and Drug Administration (FDA) on September 13th, the agency has issued 11 warning letters to multiple manufacturers and retailers for selling or distributing unauthorized e-cigarette products at industry trade shows.
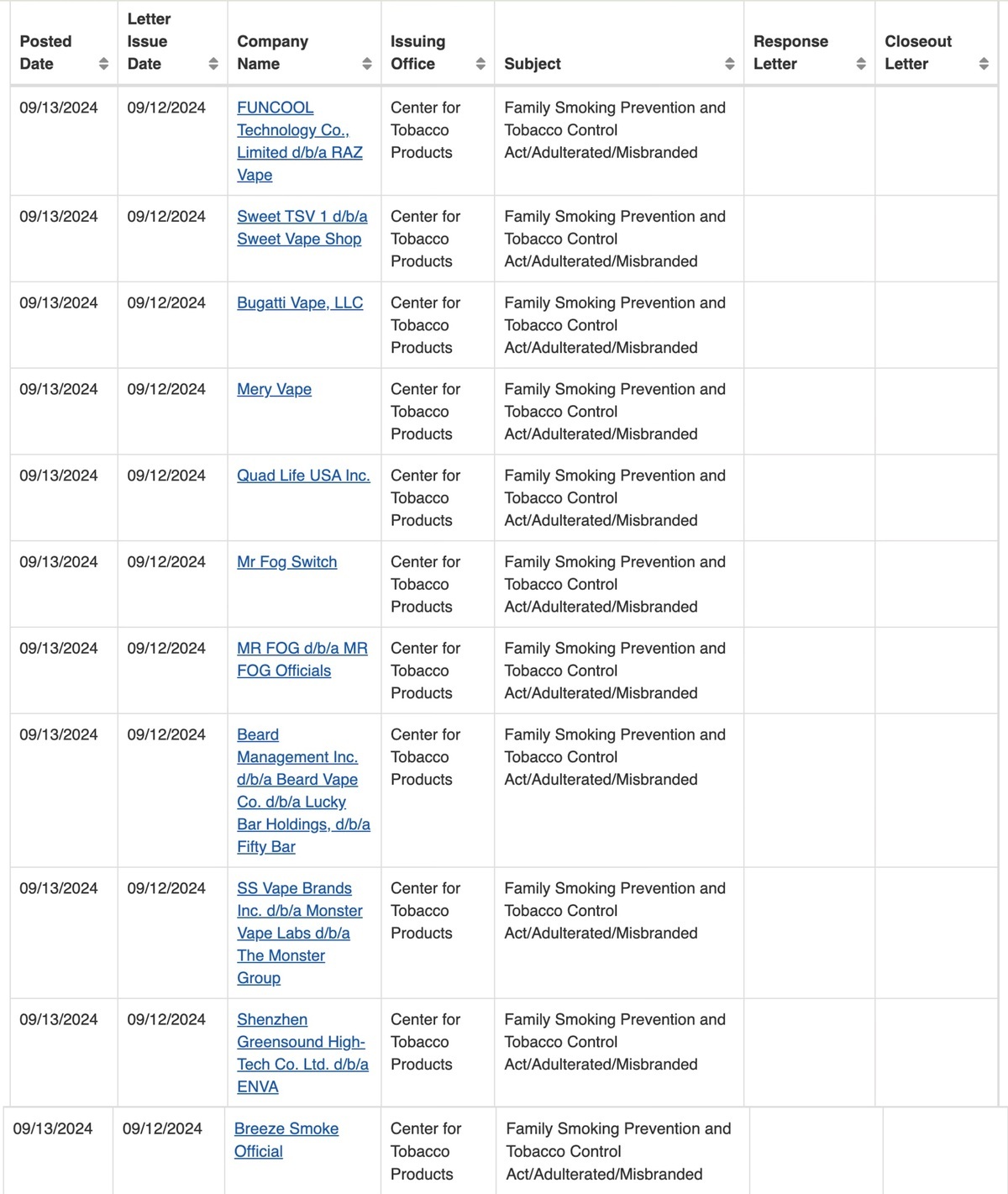
The FDA's Center for Tobacco Products (CTP) conducted an investigation after attending a trade show and found that six retailers and manufacturers were suspected of selling or distributing unauthorized e-cigarette products, violating regulations under the Federal Food, Drug, and Cosmetic Act. John Verbeten, Director of CTP's Office of Compliance and Enforcement, emphasized that the FDA will gather leads and conduct investigations through various channels, including trade shows, to crack down on illegal activities.
In addition, the FDA has issued warning letters to five online retailers accusing them of selling unauthorized e-cigarette products popular among teenagers, such as brands like Breeze, Mr. Fog, and Raz. These brands of e-cigarette products have high usage rates among teenagers and are favored for their wide variety of flavors.
Source: FDA official website.
Companies that have received a warning letter must respond within 15 business days, detailing the actions taken to address the violations and prevent future non-compliance. Companies that fail to address the issues in a timely manner may face further action by the FDA, including injunctions, seizures, or civil penalties.
The FDA's actions are part of its ongoing efforts to combat the unauthorized sale of e-cigarette products in the supply chain. Over the past year, the FDA has conducted multiple retailer inspections, exposing numerous cases of unauthorized e-cigarette sales, issuing over 690 warning letters, and imposing more than 140 civil penalties on retailers.
It is worth noting that the percentage of American teenagers using e-cigarettes decreased by nearly 25% between 2023 and 2024, dropping from 2.13 million to 1.63 million. The FDA has authorized 34 types of e-cigarette products and devices that are legally sold in the United States. The public can access further information about legally marketed tobacco products in the FDA's searchable tobacco product database. With increasing global regulations on e-cigarettes, governments around the world are taking unprecedented measures to combat illegal e-cigarette activities. From the seizure of over a thousand untaxed e-cigarettes by German customs, to the sales bans imposed by the Department of Trade and Industry in the Philippines on brands such as Relx, Flare, Team X, and Funky Monkey, these actions not only demonstrate the government's commitment to protecting public health and upholding tax regulations but also reflect the international community's collective efforts in combating illegal e-cigarette trade.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








