On October 31, 2Firsts reported that the U.S. Food and Drug Administration (FDA) issued warning letters to nine online retailers and one manufacturer, accusing them of selling unauthorized smart e-cigarettes (click to read the article). According to 2Firsts' analysis of the FDA warning letters, six e-cigarette brands were involved: FUME, Craftbox, North Vape, Posh, Halo Vapor, and Swype.
2Firsts noted that in the ten FDA warning letters, the agency described how "these products' labels or advertising may appeal to youth by imitating smartphones or other smart technologies" and "may attract teenagers by resembling gaming products," labeling these products as "highly concerning".
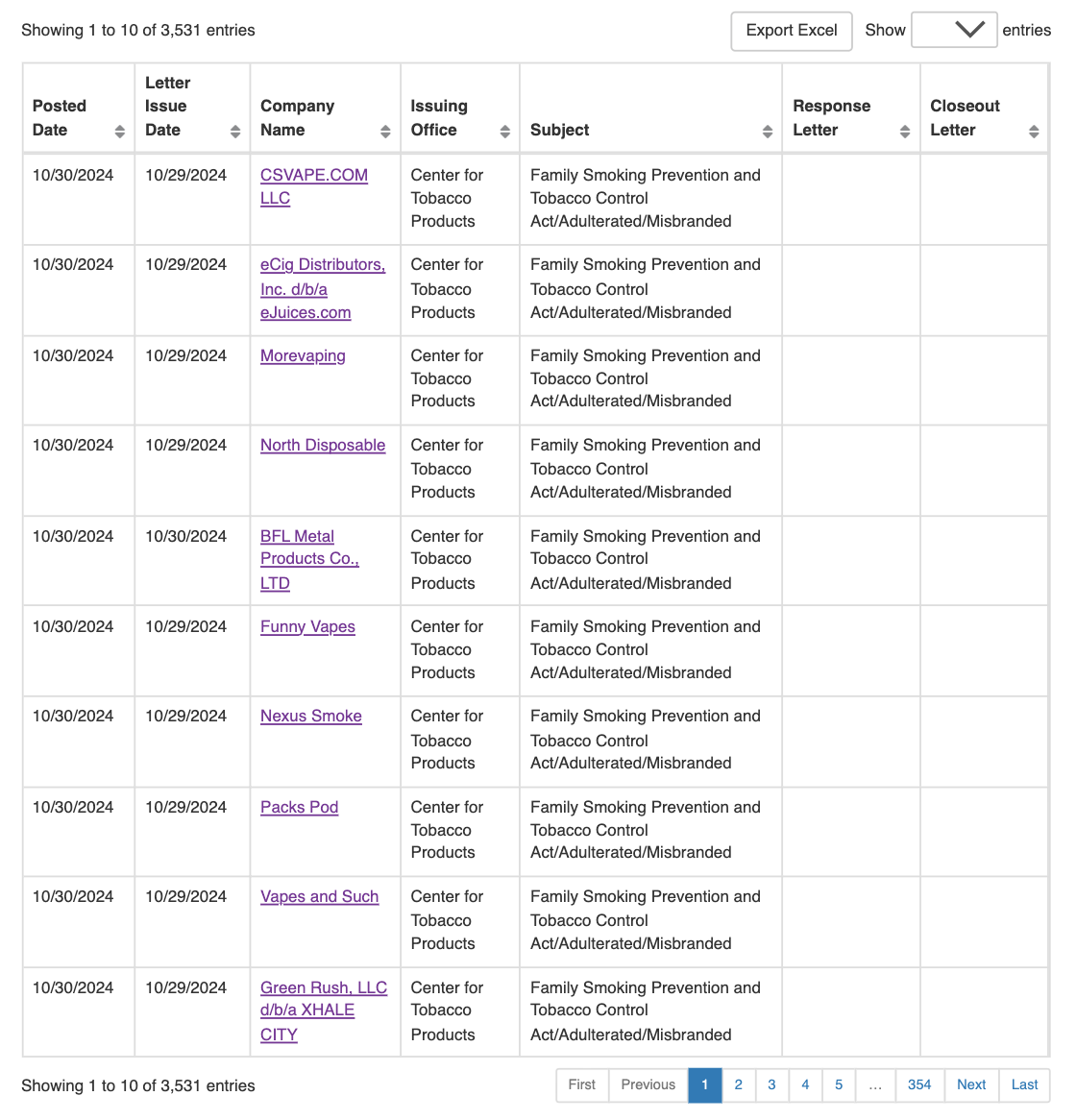
Below is a list compiled by 2Firsts from the FDA warning letters, detailing the brands and products mentioned:
FUME
The product cited in the warning letter is:
- WeFume Vape 30K Puffs – cited 3 times
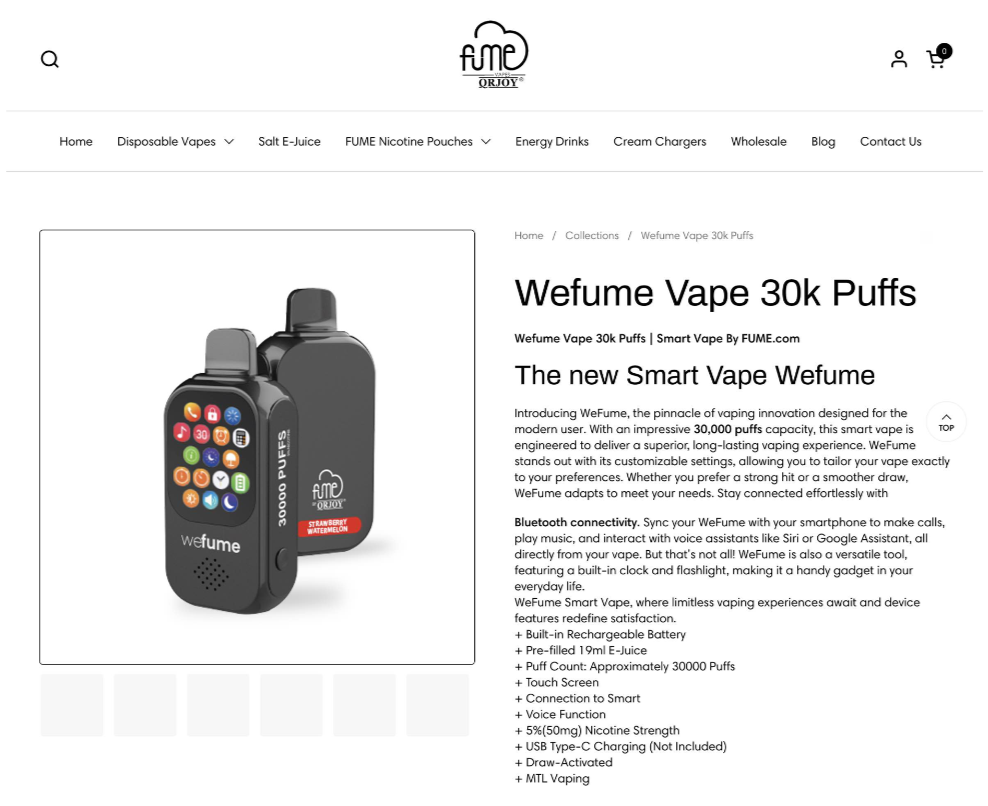
Craftbox
The products mentioned in the warning letters are:
- Craftbox V-Touch 30k Puff Smart Disposable Vape – cited 4 times
- Craftbox V-Play 20K Puffs Disposable Vape – cited 5 times
- Craftbox V-Touch 30,000 Puffs Phone Call Vape Disposable– cited 1 time

North Vape
The products cited in the warning letter are:
- North South Connect 35K Puffs Phone Disposable Vape – cited 2 times
- South Connect 35K Disposable Vape Bluetooth 900mAh – cited 1 time


Posh
The products cited in the warning letter are:
- Posh Xtron 30000 Disposable Vape – cited 1 time
- Posh Pro Max 30000 – cited 1 time

Halo Vapor
The product cited in the warning letter is:
- Halo Vapor Synix 30K – cited 2 times

Swype
The product cited in the warning letter is:
- Swype 30K Disposable Vape – cited 1 time

According to information disclosed by the FDA, when the agency identifies what it considers to be serious violations of federal requirements, it notifies the relevant parties.
This notification typically takes the form of a warning letter, which highlights issues such as poor manufacturing practices, problematic product efficacy claims, or incorrect usage instructions. The letter provides companies or individuals with an opportunity to address the FDA’s concerns, requiring a response within a specified timeframe. This response may include a corrective plan, after which the FDA will conduct follow-up inspections to ensure that corrective measures are sufficient.
If a company or individual disagrees with the FDA's assessment, they have the opportunity to present their reasons and supporting information to the FDA. These communications and other actions between the FDA and the letter recipient may alter the regulatory status of the issues discussed in the letter.
As of October 30, 2024, the FDA has authorized 34 e-cigarette products and devices. The agency maintains a printable one-page flyer listing all authorized e-cigarette products, which retailers can reference to verify which products may be legally marketed and sold in the United States. Entities manufacturing, importing, selling, or distributing e-cigarettes without the necessary premarket authorization will face enforcement risks.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







