The Food and Drug Administration said it would allow Juul Labs Inc. to stay on the U.S. market while the e-cigarette maker appeals the agency’s ban on its products.
The FDA in June ordered the vaping company to pull its e-cigarettes off the U.S. market, saying Juul hadn’t submitted sufficient evidence that they were safe. A federal appeals court then granted Juul a temporary stay of the order, and Juul was asking the court for a longer stay to continue selling its e-cigarettes for the duration of the appeal process. Juul separately had asked the FDA to stay its own order pending the appeal.
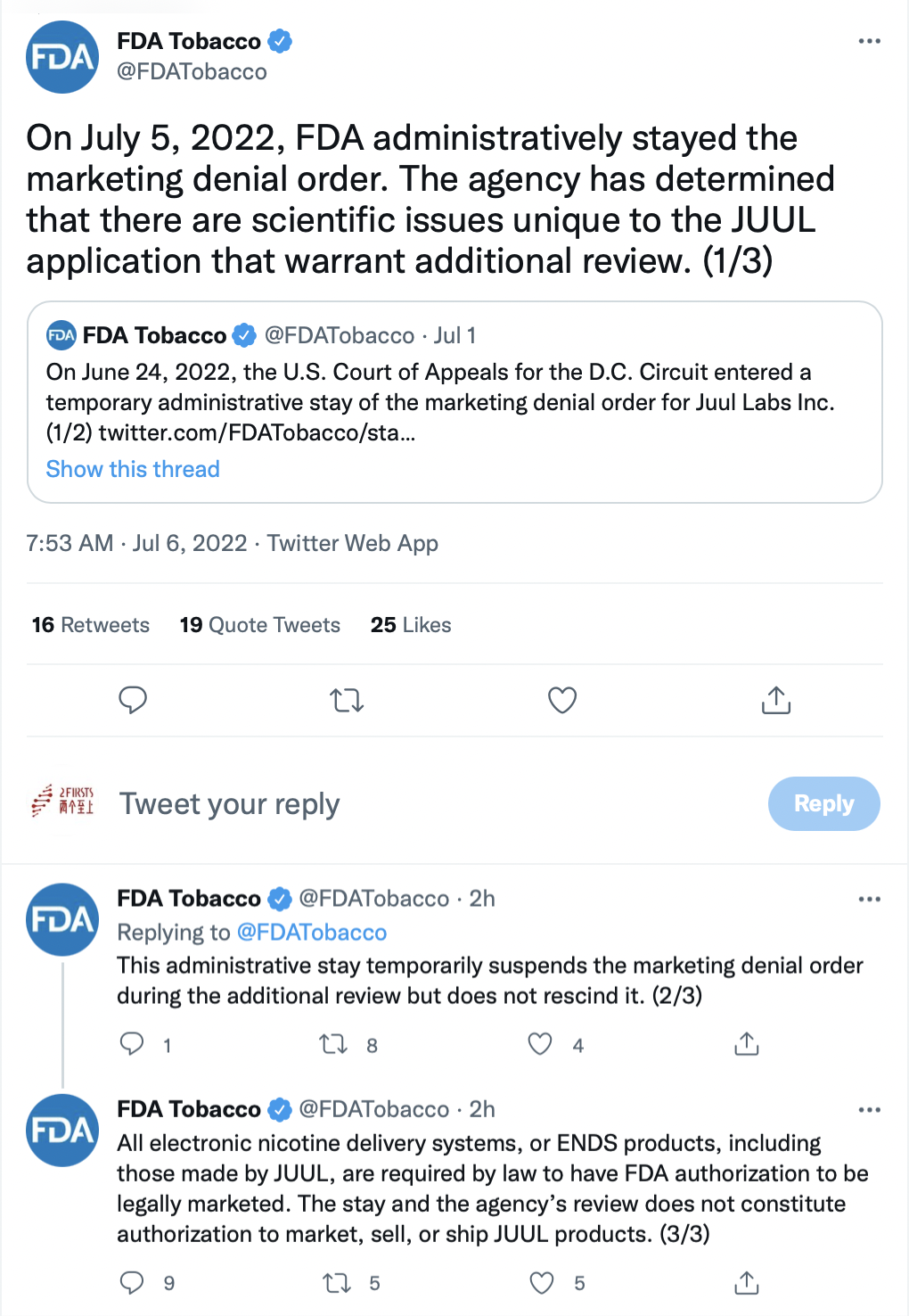
In messages posted on Twitter late Tuesday, the FDA said it had stayed its marketing denial order. “The agency has determined that there are scientific issues unique to the JUUL application that warrant additional review,” the FDA said. The administrative stay temporarily suspends the order during the additional review but doesn’t rescind it, the agency said.
The FDA initially rejected Juul’s request for a stay, prompting Juul to seek a stay of the ban in court, according to Juul’s court filings.
Juul alleged in court filings that the FDA had mishandled its application. In the court filings, Juul said the agency had overlooked more than 6,000 pages of data that the company had submitted to the FDA on the aerosols that users inhale. Juul also said in court filings that the FDA’s decision was influenced by political pressure. The FDA had until July 7 to respond to Juul’s motion in federal court.
The content excerpted or reproduced in this article comes from a third-party, and the copyright belongs to the original media and author. If any infringement is found, please contact us to delete it. Any entity or individual wishing to forward the information, please contact the author and refrain from forwarding directly from here.








