
Special announcement:

This article is for internal industry communication only and does not make any recommendations for brands or products.

The images presented in this article are used solely for factual description and are not intended as any form of product advertisement.

Minors are prohibited from accessing this article.
Firsts observed that the e-cigarette brand HIFLOW is showcasing its new e-cigarette, the MODEX Morph, on its official website. This product features two refillable pods with flavor control, which the brand claims is the "WORLD'S 1ST Dual Open Pod with Flavor Control.
More specifically, the product specifications of HIFLOW MODEX Morph are shown in the table below:

In terms of product features, the main innovation of HIFLOW MODEX Morph lies in its inclusion of a 1.5ml auxiliary pod and a 2ml main pod, both of which have an open design. The brand states that users can fill each pod with their preferred ice-flavored e-liquid, sweet e-liquid, and flavored e-liquid based on personal preference.
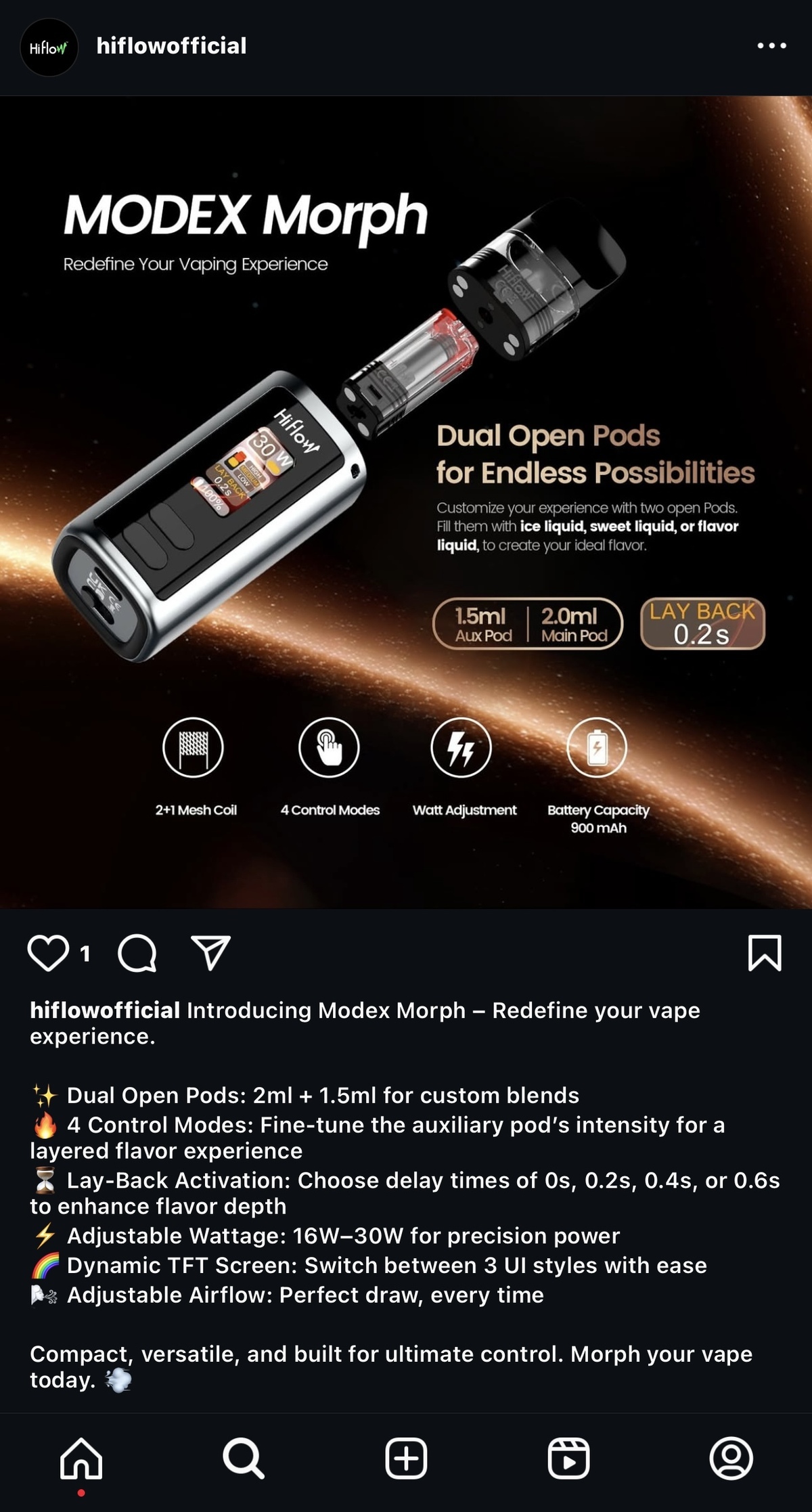
The main pod offers options of 0.6Ω and 0.8Ω, and the e-liquid in the main pod will be heated by dual core mesh, while the e-liquid in the auxiliary pod will be heated by single core mesh.
It is worth noting that both the main pod and auxiliary pod of HIFLOW MODEX Morph support refilling e-liquid up to 5 times.
In addition, the HIFLOW MODEX Morph also features a "delayed activation" function, with the brand stating that the product can offer different experiences when activated after delays of 0, 0.2, 0.4, or 0.6 seconds.
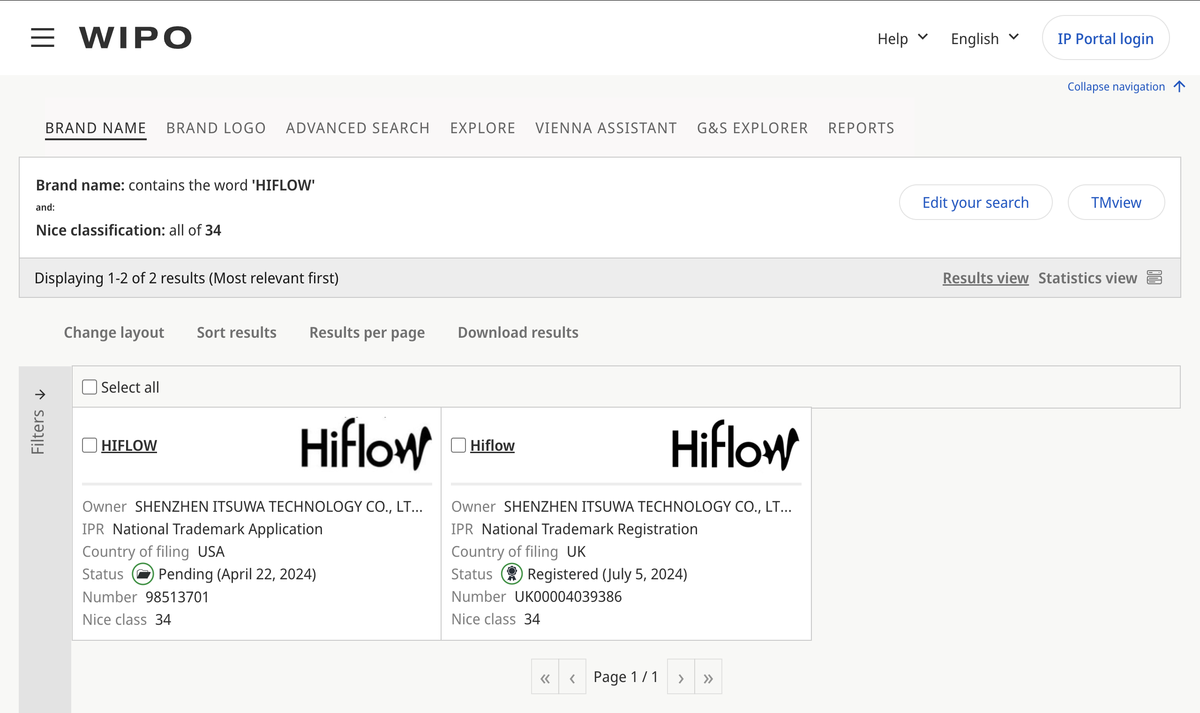
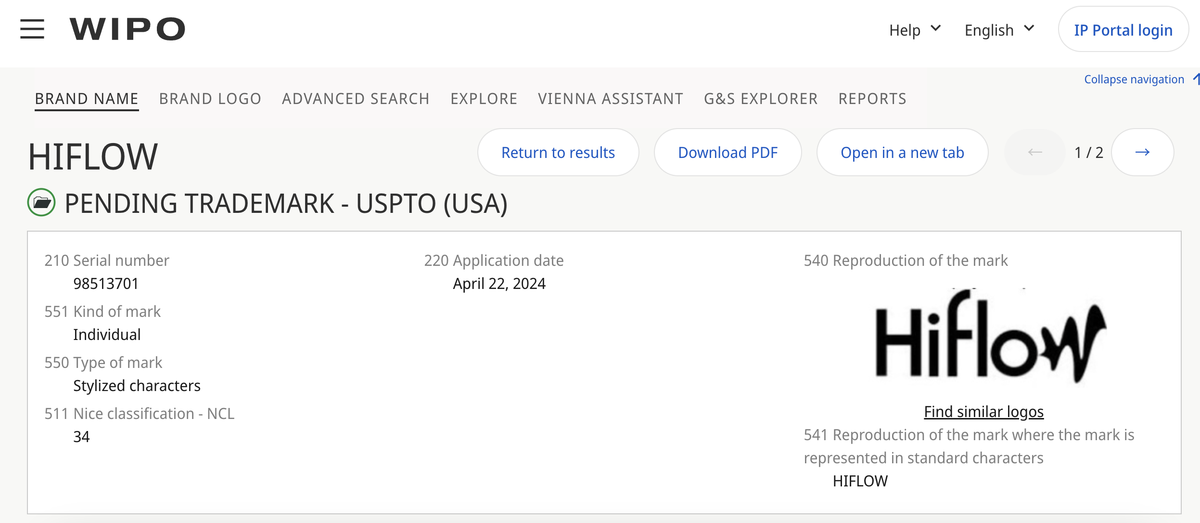
According to a search on the official website of the World Intellectual Property Organization (WIPO), the "HIFLOW" brand trademark has been registered by SHENZHEN ITSUWA TECHNOLOGY CO., LTD, also known as Shenzhen Itsuwa Corporation, in both the UK and the US regions.
According to reports, Itsuwa was founded in 2011 with its headquarters located in Baoan District, Shenzhen, Guangdong Province. The company also owns e-cigarette brands such as VOOM and Vapesoul.
Currently, the HIFLOW MODEX Morph has been launched on the official HIFLOW brand website and official social media accounts, but product sales information related to it has not yet been found on overseas e-cigarette distributor websites.
The "2FIRSTS" product column is dedicated to providing readers with the latest information on new products in the tobacco industry. To gather and share firsthand information within the industry, 2FIRSTS welcomes readers to submit articles and share the latest products in the e-cigarette field.
If you have any unique insights or information, feel free to contact us anytime via email at info@2firsts.com, or connect with 2Firsts CEO Alan Zhao on LinkedIn.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com








