
Key points:
1.The Health Service Executive (HSE) in the UK and Ireland has issued an urgent safety warning regarding three e-cigarette products that were incorrectly labeled as "nicotine-free" when they actually contain 18.0 to 19.0 mg/ml of nicotine.
2.The nicotine e-liquid capacity of these products ranges from 7.7ml to 9.4ml, far exceeding the 2ml limit imposed by Irish law.
3.The HSE has reported this issue to the European Union's RAPEX rapid alert system and has requested retailers to immediately remove the related products from shelves. Consumers are advised to stop using the products and to return them to the place of purchase.
4.The HSE emphasizes that retailers and importers need to strengthen their supply chain management to ensure product compliance and avoid legal liability.
5.The Health and Safety Executive will continue to use its legal powers, including product seizure, destruction, and prosecution, to protect public health.
According to The Sun's report on May 12, the Health Service Executive (HSE) in Ireland recently issued an urgent safety warning for three e-cigarette products being sold in the Irish market. The products were incorrectly labeled as "nicotine-free," but actually contain high levels of nicotine.
According to analysis by the national laboratory, the following product was found to contain a nicotine concentration of 18.0 to 19.0 mg/ml. Although it did not exceed the legal limit of 20 mg/ml in Ireland, the discrepancy between this and the "nicotine-free" label is clear.
·Crystal Bling offers 6000+ puffs, in the sub-brand (flavor) 5G HRTP Blue Razz Lemonade, with batch number THE240801.
·McKesse MK Bar 7000: The sub-brand (flavor) Passionfruit & Lime is also available.
·JNR Crystal Pro Max provides 5000+ puffs, labeled as 0% nicotine, in the sub-brand (flavor) Kiwi Watermelon Ice, with batch number C24H8399-CP5000.
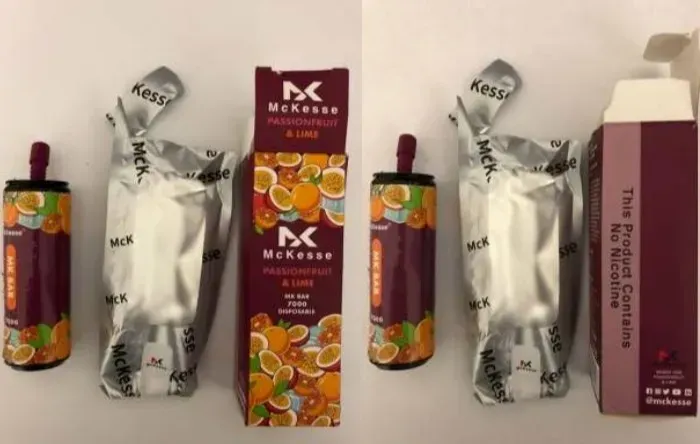


In addition, the nicotine e-liquid capacity of these products ranges from 7.7ml to 9.4ml, far exceeding the 2ml limit set by Irish law.
The HSE has reported this issue to the European Rapid Alert System (RAPEX), requesting retailers to immediately remove the related products from shelves and provide information on the products and their traceability.
The Health and Safety Executive (HSE) stated that retailers who have sold or distributed these products must prominently display the recall notice in their stores, on their websites, and on social media.
The Chief Environmental Health Officer for the HSE region, Maurice Mulcahy, has stated that while the nicotine concentration of these products does not exceed the legal limit, their labeling is misleading to consumers and the nicotine e-liquid content greatly exceeds the standard, posing a safety hazard. This combination of inaccurate labeling and excessive e-liquid content represents a dual violation that poses a threat to public health. In order to prevent further harm, the HSE will take legal action, including but not limited to seizing and destroying the products and initiating legal proceedings against those responsible.
He also reminded retailers to fulfill their legal responsibilities by verifying the qualifications of suppliers and the compliance of products before selling them. He urged all e-cigarette retailers and importers to ensure that the products they sell have gone through the necessary notification process in the European Union Common Entry Gate (EU CEG), especially for nicotine products imported from countries outside the EU, such as the UK, they should comply with additional declaration obligations from the importing party.
We welcome news tips, article submissions, interview requests, or comments on this piece.
Please contact us at info@2firsts.com, or reach out to Alan Zhao, CEO of 2Firsts, on LinkedIn
Notice
1. This article is intended solely for professional research purposes related to industry, technology, and policy. Any references to brands or products are made purely for objective description and do not constitute any form of endorsement, recommendation, or promotion by 2Firsts.
2. The use of nicotine-containing products — including, but not limited to, cigarettes, e-cigarettes, nicotine pouchand heated tobacco products — carries significant health risks. Users are responsible for complying with all applicable laws and regulations in their respective jurisdictions.
3. This article is not intended to serve as the basis for any investment decisions or financial advice. 2Firsts assumes no direct or indirect liability for any inaccuracies or errors in the content.
4. Access to this article is strictly prohibited for individuals below the legal age in their jurisdiction.
Copyright
This article is either an original work created by 2Firsts or a reproduction from third-party sources with proper attribution. All copyrights and usage rights belong to 2Firsts or the original content provider. Unauthorized reproduction, distribution, or any other form of unauthorized use by any individual or organization is strictly prohibited. Violators will be held legally accountable.
For copyright-related inquiries, please contact: info@2firsts.com
AI Assistance Disclaimer
This article may have been enhanced using AI tools to improve translation and editorial efficiency. However, due to technical limitations, inaccuracies may occur. Readers are encouraged to refer to the cited sources for the most accurate information.
We welcome any corrections or feedback. Please contact us at: info@2firsts.com







